Int. J. Dev. Biol. 66: 187 - 197 (2022)
Special Issue: Developmental Biology in Greece
Lipid rafts integrity is essential for prolactin-induced mitogenesis in mouse embryonic stem cells
Original Article | Published: 22 November 2021
Abstract
Embryonic stem cells, ESCs, retain the capacity to self-renew, yet, the protein machinery essential in maintaining this undifferentiated status remains largely undefined. Signalling interactions are initiated and enhanced at the plasma membrane lipid rafts, within constraints and regulations applied by the actin and tubulin cytoskeleton systems. First, we undertook a comprehensive approach using two-dimensional gel electrophoresis and mass spectrometry analysis combined with Western blotting and immunofluorescence analyses at the single cell level to compile the proteome profile of detergent-free preparations of lipid rafts of E14 mouse embryonic stem cells. In comparison with the proteomic profiles of other membrane fractions, recovery of actin and tubulin network proteins, including folding chaperones, was impressively high. At equally high frequency, we detected annexins, pleiotropic proteins that may bind membrane lipids and actin filaments to regulate important membrane processes, and we validated their expression in lipid rafts. Next, we tested whether lipid raft integrity is required for completion of mitogenic signalling pathways. Disruption of the rafts with the cholesterol sequestering methyl-β-cyclodextrin (MCD) greatly downregulated the mitotic index of ESCs, in a dose- and time of exposure-dependent manner. Moreover, MCD greatly reduced the mitogenic actions of prolactin, a hormone known to stimulate proliferation in a great variety of stem and progenitor cells. Taken together, our data postulate that lipid rafts in ESCs act in close association with the actin and tubulin cytoskeletons to support signal compartmentalization, especially for signalling pathways pertinent to symmetric divisions for self-renewal.
Keywords
lipid rafts, annexin, prolactin, stem cells
Introduction
Embryonic stem cells (ESCs) from the inner cell mass of embryos at the blastocyst stage are capable of unlimited, symmetrical, self-renewing mitoses when established as cell lines in culture (Burdon et al., 2002). However, when re-introduced into a blastocyst, ESCs retain their ability to differentiate into all three embryonic germ layers and generate an embryo (Martin 1980; Brook and Gardner 1997; Thomson and Marshall 1998; Burdon et al. 2002). Therefore, as ESCs may provide a renewable cell source for a diversity of conditions, such as traumas, diabetes, myocardial infarction, or neurodegeneration, considerable effort has been made to understand the mechanisms that maintain pluripotentiality or guide ESCs along specific cell lineages. We now know that activation of the Leukaemia Inhibitory Factor (LIF)/Janus kinase 2 (JAK2)/Signal Transducer and Activator of Transcription Protein 3 (STAT3) pathway is required to maintain the undifferentiated state of ESCs, as well as certain levels of activation of Protein kinase C, TGFβ/SMAD, Wnt/β-catenin, and FGF/MEK/ERK pathways, with low levels of ERK activation essential for telomere length maintenance and genomic stability (Chen et al. 2015; Huang et al. 2015; Ma et al. 2016). Despite these extensive studies, we still know little about the subcellular structures involved in the facilitation of signalling complex formation and intracellular propagation of the signal.
Accumulated experimental evidence has highlighted the importance of coalescing lipid rafts as signalling platforms, formed within constrains applied by the underlying cortical filamentous actin (F-actin) cytoskeleton and its associated proteins (Gupta et al. 2006; Morone et al. 2006; Chichili and Rodgers 2007; Goswami et al. 2008; Simons and Gerl 2010; Asimaki et al. 2011; Asimaki and Mangoura 2011; Karouzaki et al. 2019). Lipid rafts represent pre-ordained, liquid-ordered, cholesterol-rich microdomains of the plasma membrane bilayer, with glycosphingolipids and glycosyl phosphatidylinositol–anchored proteins in their outer leaflet, integral transmembrane, palmitoylated proteins (mostly receptors and transmembrane kinases, like JAKs) (Ren et al. 2013; Lorent et al. 2017), and high concentrations of major lipidated signalling molecules, such as the lipid raft prototype resident H-Ras, Src-family kinases, G proteins, and adaptor molecules in their inner leaflet and cytoplasmic face (e.g., (Asimaki et al. 2011; Karouzaki et al. 2019)). These specialized concentrations contribute, through lipid-lipid and lipid-protein interactions (Raghupathy et al., 2015), to the generation and dynamic behavior of lipid raft domains (reviewed in (Harayama and Riezman 2018; Kusumi et al. 2020)).
More specifically, in quiescent cells, lipid rafts exist as meso-scale domains of 2-20 nm diameter, which float amidst liquid-disordered plasma membrane domains. Upon binding of clustering agents (ligands, antibodies, or toxins, e.g., (Puri et al. 2005; Dinic et al. 2015)) to their transmembrane receptors, the clustered receptors induce lipid rafts to coalesce and form larger, stabilized membrane domains (up to 300 nm). The liganded receptors might be clustered for longer durations, yet their lipid-anchored signalling effectors move freely and undergo rapid exchanges with those in the lipid-disordered domains. Therefore, the raft domains now function as platforms that facilitate formation of signalling complexes and biomolecular condensates upon membrane receptor stimulation (Simons and Sampaio 2011; Kusumi et al. 2012).
Indeed, the significance of biomolecular condensates (i.e. concentrations of macromolecules not surrounded by a membrane) in the biological outcomes of membrane receptor signalling has now become evident (Asimaki and Mangoura 2011; Mangoura 2016; Banani et al. 2017; Shin and Brangwynne 2017; Karouzaki et al. 2019; Jaqaman and Ditlev 2021; Schuster et al. 2021). Such condensates, formed at the plasma membrane upon receptor agonist binding, regulate the activity of signalling proteins, by regulating the time that such molecules spend in the membrane (Huang et al., 2019). Furthermore, these condensates also control actin assembly and remodelling (Case et al., 2019).
While actin cortical structures are remodelled by biomolecular condensates, they also control the lateral movement of lipid rafts (Simons and Gerl, 2010). Specifically, constraints are imposed by F-actin and crosslinking proteins that act as fences, and by interacting proteins, namely cytosolic domains of transmembrane proteins or cytosolic proteins tethered to the membrane through lipid modifications, which act as pickets (Kusumi and Suzuki 2005; Kusumi et al. 2012). This extensive network of “fences” and “pickets” forms restraining “corrals” for the movement of lipid rafts through the entirety of the plasma membranes. As F-actin binding proteins are instantly modified by receptor-mediated activation of signalling kinases, the fate of signalling complexes (e.g., (Chen et al. 2009; Rollason et al. 2009)) and, overall, the spatiotemporal activity of signalling pathways is controlled.
Alpha and beta tubulins, resident proteins in raft microdomains (Ballestrem et al. 2000; Li et al. 2004), through physical interactions with lipid raft residents flotillin 1, caveolin, and G-proteins are also important functional partners of lipid rafts (Li et al. 2004; Dremina et al. 2005; Allen et al. 2009). Microtubules (MTs), the polymerized dimers of α- and β-tubulin, are highly dynamic in the cell periphery, and the rapid interchange between elongation and catastrophe is modulated by a number of molecules, mostly microtubule associated proteins (MAPs), within or in close association with lipid rafts. These roles of microtubules and rafts as reciprocal scaffolds have become a focus for the lipid raft role in signal-induced, clathrin-independent endocytosis (Chen and Williams, 2013), and in cell motility and neuritic process outgrowth (Xu et al., 2011). Thorough identification of the molecular protein component of rafts would greatly facilitate these efforts.
Lipid rafts have previously been implicated in embryonic and adult stem cell renewal and expansion (Androutsellis-Theotokis et al. 2010; Lee et al. 2010) and appear to have particular properties. For example, human ESCs are insensitive to cholera toxin that targets the monosialotetrahexosylganglioside-containing lipid rafts and Gs-proteins, but sensitive to pertussis toxin that targets Gi-proteins (Nakamura et al., 2009). These studies have highlighted the importance of lipid rafts in membrane receptor regulation of ESC self-renewal. However, the protein composition of lipid rafts remains elusive in ESCs (Nagano et al. 2005; Wang and Gao 2005; Intoh et al. 2009). Therefore, we have sought to analyse the proteome of lipid rafts in two murine ES cell lines - E14 and W4 - as well as to test the functional importance of lipid raft integrity for the mitogenic signalling of prolactin (PRL), an established mitogen for many stem and progenitor cells, including precursor cells in the adult mouse hippocampus (Walker et al., 2012), astroblasts and neuroblastoma cells (Cheng et al. 2000; Mangoura et al. 2000), as well as many cancer stem cell types (e.g., (Rouet et al., 2010)).
Results and Discussion
The proteome profile of lipid rafts from stem cells reveals enrichment in tubulins, actins, and annexin
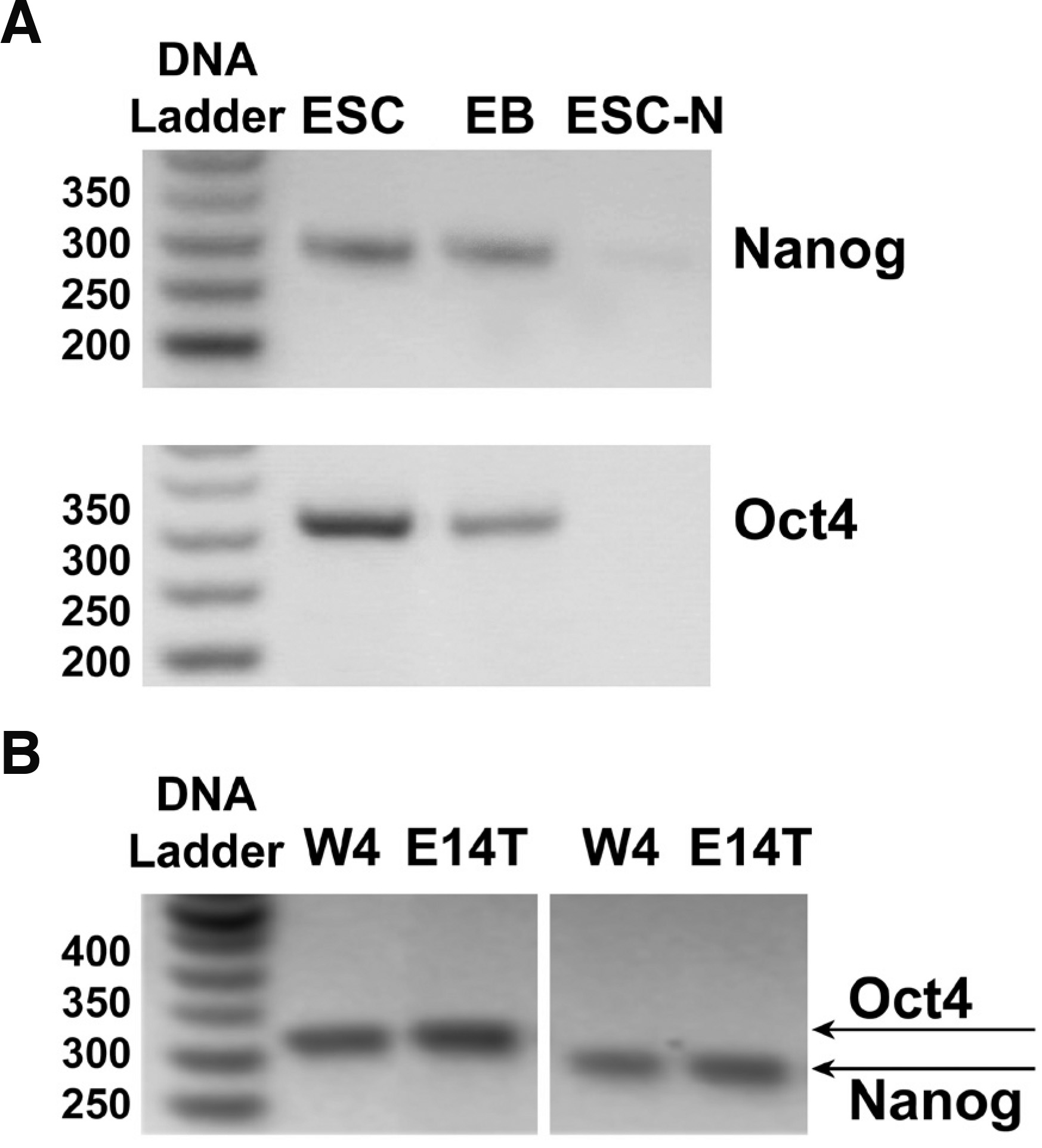
E14T or W4 ES cells grew with a cell cycle of 18-24 h for the first 3-days post plating, and then mitosis rates fell, most likely due to silencing of bivalent chromatin domains that silence developmental gene, while keeping cells poised for activation upon initiation of differentiation (Bernstein et al., 2006); thus, cultures were usually passaged after 96 h. Both mouse ESC lines (mESC) were routinely monitored by morphology and by semiQ-PCR of stemness markers Nanog and Oct-4 (Fig. 1) to verify their undifferentiated state (McLaughlin et al., 2006), as these transcription factors control pathways that govern pluripotency, self-renewal, genome surveillance and cell fate determination (Loh et al., 2006). Under these conditions, ESCs could be induced to form embryoid bodies (EBs, Fig. 1) and, after treatment of EBs with retinoic acid and replating, to differentiate to neurons (Fig. 1, ESC-N), using established protocols (Bibel et al. 2004; McLaughlin et al. 2006). As shown in Fig. 1, both Nanog and Oct-4 expression was downregulated in EBs and practically nullified in differentiated neurons. In order to have a nearly synchronized cell population and thus maximum cell protein content homogeneity, we analyzed the proteome of lipid rafts at 60 h post-plating.
Fig. 1. Expression of stem cell markers in mouse stem or differentiated cells.
(A) mRNA expression levels of Nanog (285 bp) or Oct4 (313 bp), determined by semi-quantitative PCR as described in Methods, is high in E14 ES cells (lanes ESCs); expression of both stemness markers was significantly decreased when ESCs were withdrawn from LIF and induced to form embryoid bodies (lanes EBs), and became undetectable when EBs were neuronally induced with retinoic acid (lanes ESC-N). (B) Identical results were obtained with the mESC line W4.
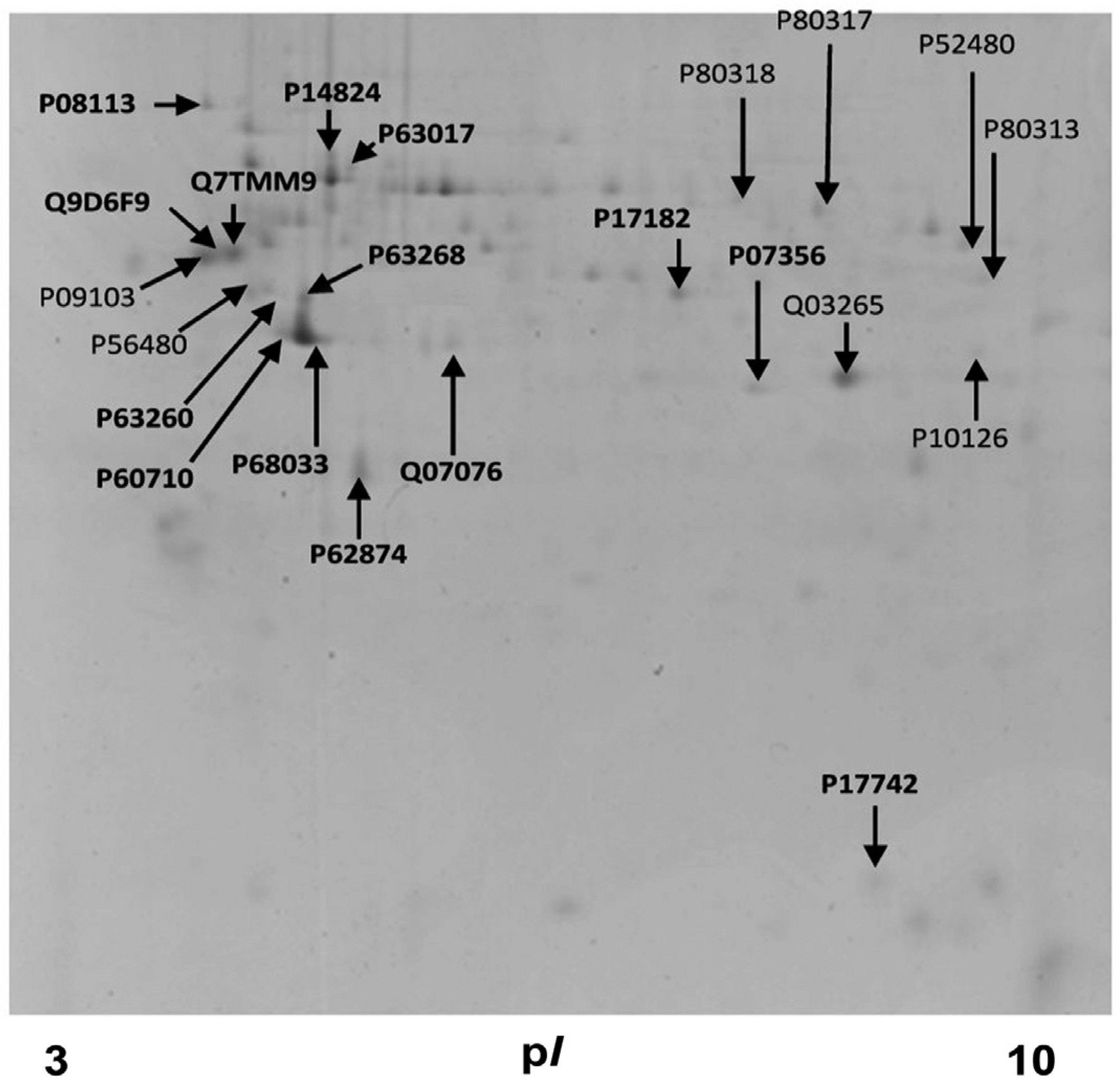
Using an optimized detergent-free method that preserves the native lipid and protein composition of the lipid rafts, and allows considerable resolution of lipid rafts, from caveolin-rich domains, and intracellular ER or Golgi membranes (Chambers et al. 2003; Asimaki et al. 2011; Mangoura 2016; Karouzaki et al. 2019), we analyzed enrichments of lipid raft proteins in E14T and W4 ESCs (Fig. 2). Protein content is very low compared with other fractions (e.g., Fig. 3 and (Karouzaki et al., 2019)) and therefore we identified and analyzed all visible spots, i.e. 81 spots from 2-DE gels at pH 3-10, 66 of which were positively identified and 28 found to be unique in the lipid raft fractions, as compared with the rest of the membrane fractions (pooled fractions 1-5, Table 1). The ratio of structural proteins was impressively high, as previous studies have also noted (Nagano et al., 2005). Indeed, recovery of actin and tubulin network proteins was truly significant (Table 1). Moreover, the presence of almost all actin isoforms and of T-complex subunits (P80318, P80313, P80317), which are well known molecular chaperons for folding of actin and tubulin, shows that we have isolated both the outer and inner leaflet of lipid rafts, as well as the intimately associated underlying cortical cytoskeleton. This task is almost impossible when detergents are used and these components get separated (Li et al. 2004; Morris et al. 2004; Persaud-Sawin et al. 2009).
Fig. 2. Representative two-dimension gel image of proteins from E14 stem cell lipid rafts.
Proteins from detergent-free preparations of lipid rafts (pooled fractions 1-5), were separated with SDS-PAGE on 12% polyacrylamide gel and stained with Coomassie Brilliant Blue. Arrows indicate protein spots that were processed and the accession number and position of identified proteins.
Table 1
Mouse Embryonic Stem Cells proteins detected with MS in detergent-free lipid raft preparations
| Uniprot ID | Protein Full Name | Gene Name |
| P62737 (ACTA_MOUSE) | Actin, aortic smooth cell | Acta2 |
| P60710 (ACTB_MOUSE) | Actin, cytoplasmic 1 | Actb |
| P68033 (ACTC_MOUSE) | Actin, α cardial muscle | Actc1 |
| P63260 (ACTG_MOUSE) | Actin, cytoplasmic 2 | Actg1 |
| P63268 (ACTH_MOUSE) | Actin, γ enteric smooth | Actg2 |
| P07356 (ANXA2_MOUSE) | Annexin A2 | Anxa2 |
| P14824 (ANXA6_MOUSE) | Annexin A6 | Anxa6 |
| Q07076 (ANXA7_MOUSE) | Annexin A7 | Anxa7 |
| Q03265 (ATPA_MOUSE) | ATP synthase subunit α, mitochondrial | Atp5f1a |
| P56480 (ATPB_MOUSE) | ATP synthase subunit β, mitochondrial | Atp5f1b |
| P10126 (EF1A1_MOUSE) | Elongation Factor1 α1 | Eef1a1 |
| P08113 (ENPL_MOUSE) | Endoplasmin | Hsp90b1 |
| P62874 (GBB1_MOUSE) | Guanine nucleotide-binding protein, b1 subunit | Gnb1 |
| P63017 (HSP7C_MOUSE) | Heat shock cognate 71kDa protein | Hspa8 |
| P17742 (PPIA_MOUSE) | Peptidyl-propyl-cys-trans isomer | Ppia |
| P09103 (PDIA1_MOUSE) | Protein Disulfide-isomerase | P4hb |
| P52480 (KPYM_MOUSE) | Pyruvate kinase isozymes M1/M2 | Pkm |
| Q7TMM9 (TBB2A_MOUSE) | Tubulin β-2A chain | Tubb2a |
| Q9CWF2 (TBB2B_MOUSE) | Tubulin β-2B chain | Tubb2b |
| P68372 (TBB4B_MOUSE) | Tubulin β-2C chain | Tubulin β-2C chain |
| Q9ERD7 (TBB3_MOUSE) | Tubulin β-3 chain | Tubb3 |
| Q9D6F9 (TBB4A_MOUSE) | Tubulin β-4A chain | Tubb4a |
| Q3TFB6 (TBB5_MOUSE) | Tubulin β-5 chain | Tubb5 |
| Q922F4 (TBB6_MOUSE) | Tubulin β-6 chain | Tubb6 |
| P80318 (TCPG_MOUSE) | T-complex protein 1 subunit γ | Cct3 |
| P80313 (TCPH_MOUSE) | T-complex protein 1 subunit η | Cct7 |
| P80317 (TCPZ_MOUSE) | T-complex protein 1 subunit ζ | Cct6a |
Actins and Tubulins
The actin cytoskeleton is indispensable for all primal cell processes, i.e. mitosis, cell motility and migration, and regulation of signalling complexes, as is also attested by the fact that mRNAs for β and γ actin are the most abundant species during oogenesis, even increasing between the 8-cell and the blastocyst stage. Hence, mutations of actin genes are lethal in early embryonic stages (Taylor and Piko 1990; Schoenenberger et al. 2011). Out of the six actin genes and corresponding proteins (Perrin and Ervasti, 2010), five were present in the lipid rafts of mESCs (P60710, P63268, P68033, P62737, P63260). Four are considered muscle specific, out of which only Acta 1, a skeletal muscle actin, was not detected, while the cytoplasmic, ubiquitously expressed β- and γ1-actin (Actb and Actg1) isoforms were present. The abundance of actin species may signify a strong compartmentalization of the rafts in the plasma membrane of mESCs.
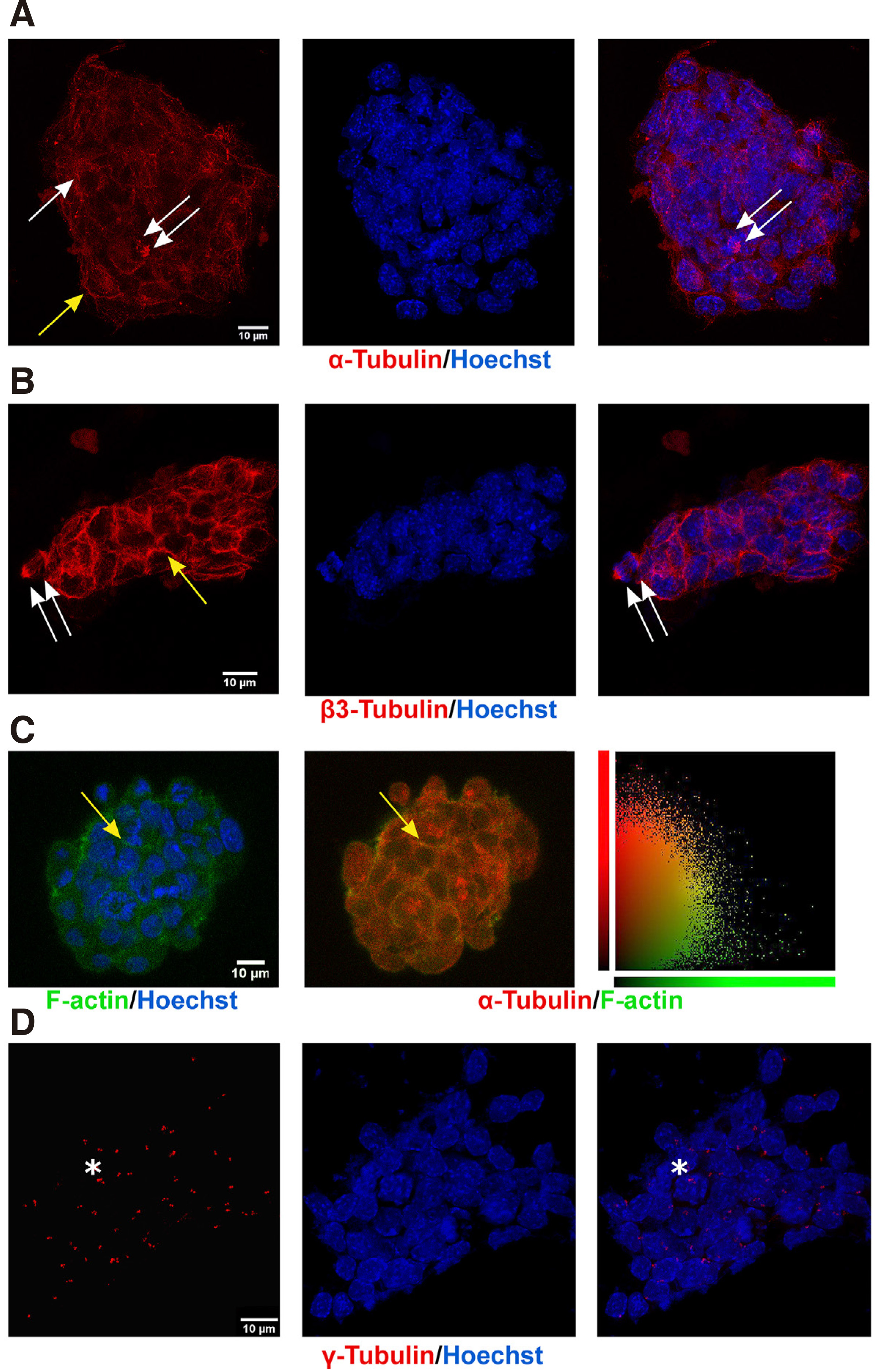
Equally crucial for such pivotal functions and general cell and organism growth right from the zygote stage are the tubulins, which serve such functions courtesy of their unique ability rapidly to form highly dynamic noncovalent polymers, the microtubules, i.e. polymerized structures of α- and β-tubulin dimers ((Brouhard and Rice, 2018), and refs therein). Both α- and β-tubulin exist as multiple isotypes, encoded by nine different genes each that have several differences in their amino acid sequences (https://www.genenames.org/); these α- and β-tubulins can form dimers (Ludueña, 2008) in a 1:1 stoichiometry (Montecinos-Franjola et al., 2019) and then, in a GTP-dependent manner, polymerize to form protofilaments that eventually assemble into the functional microtubule, i.e. the hollow, 13 protofilament-tube (Mitchison and Kirschner 1984; Chaaban and Brouhard 2017). The way in which different tubulin isotypes assemble to form specialized MT structures, given that the different post-translational modifications (PMTs) imprint on them an additional level of complexity, is now referred to as the “tubulin code”, and major research efforts are dedicated to resolving this for each function served, i.e. from mitotic spindle formation to neuronal axon transport (Ferreira et al. 2018; Janke and Magiera 2020). For these reasons, tubulins are notoriously difficult to detect proteomically, requiring in-solution digestion and detection with HPLC-MS/MS (Li et al., 2004), yet our protocols successfully detected at least most β isotypes. Alpha tubulins were not detected proteomically, as those have different trypsinization profiles, while their PMTs, such as the unique acetylation on Lys40 (Portran et al., 2017), may have interfered. Nevertheless, immunocytochemistry with an antibody that recognizes all α-tubulins (Fig. 4A) decorated MTs (in a pattern identical to the one seen with an antibody that recognizes all beta tubulins, data not shown) in the cell periphery (Fig. 4A, yellow arrow), the cytosol, and the spindle (Fig. 4A single and double arrows, respectively).
The subtypes of β-tubulin identified were β2A, β2B, β2C, β3, β4, β5, β6, (Fig. 2 and Table 1, Q7TMM9, Q9CWF2, P68372, Q9D6F9, Q3TFB6, Q922F4). Beta 2 Tubulins are highly expressed in neurons (Leandro-Garcia et al. 2010; Breuss et al. 2017; Hausrat et al. 2021), and we detected all three forms: β2A, β2B, and β2C. Beta 2 Tubulins were previously thought to overlap functionally; recent genotype-phenotype correlations, however, describe discrete human tubulinopathies (Sferra et al. 2018; Schmidt et al. 2021; Schroter et al. 2021). These, along with different phenotypes after mouse isotype-gene editing (Bittermann et al., 2019), support novel functional differences of the isotypes (Schmidt et al., 2021). The β3 isotype forms very dynamic MTs and was originally thought to be exclusively neuronal, but its high expression is now considered to be both a cancer stem cell marker and a marker of drug (taxane) resistance of solid tumor cells (e.g., (Sekino et al., 2020)). Hence, β3 may possibly participate in the typical epithelial-to-mesenchymal transition of cancer cells and in cancer metastasis processes (Sobierajska et al., 2016). Moreover, β3 is highly expressed in avian neural crest cells (Chacon and Rogers, 2019), the transient, stem-like cell population which gives rise to a range of cell types, from peripheral neurons to melanocytes. In ESCs, β3 tubulin was detected immunocytochemically in unattached microtubules in the cell periphery (Fig. 4B, yellow arrow) but not in microtubule organizing centres, and decorated the mitotic microtubules (Fig. 4B, double arrows). Indeed, the rapid dynamics of β3 would be very useful in the mitotic spindle, which assembles and de-assembles in very short times frames (< 40 min (Araujo et al., 2016)) in these rapidly dividing cells. Therefore, expression of β3 in ES cells may reflect its role in serving ESC stemness and high proliferation rate. Finally, it is noteworthy that β1-tubulin, an isotype with very low affinity for the actin cytoskeleton, was not detected.
Thus, lack of detergent in our preparations allowed the preservation of protein-protein physical interactions of tubulins with actin filaments (Tsvetkov et al., 2007), an event we also detected with immunocytochemistry, confocal imaging, and colocalization quantification in single focal planes using Volocity, as described in Materials and Methods (Fig. 4C). Indeed, the average colocalization coefficient of 0.288 + 0.05 of actin and alpha tubulin at the cell periphery (Fig. 4C, yellow arrow and graph) is comparable to those with detergent (Peta et al., 2020). The actin cytoskeleton is involved in defining the trajectory of microtubules through a family of microtubules plus-end–tracking proteins (+TIP proteins) such as CLASPs and members of the spectraplakin protein family, such as ACF7 (Kodama et al. 2003; Tsvetkov et al. 2007). Other proteins may also contribute to the presence of tubulins in the rafts, such as caveolin-1, a scaffold protein that resides in the inner leaflet of some raft-like domains (Fig. 3). Overexpression of caveolin leads to increased polymerization of microtubules by inhibition of stathmin (Kawabe et al., 2006), and its low expression in ESCs (Fig. 3A) may also indicate that the microtubule network that underlies ESC lipid rafts is highly dynamic.
Fig. 3. Annexin 2 is highly enriched in ESC lipid rafts.
(A) Subcellular fractions were prepared without detergent, as described in Materials and Methods, and 50µl of each of the 14 OptiPrep gradient fractions in Laemmli buffer was analyzed by SDS-PAGE and Western blotting; frames contain scans of typical Western blots with indicated antibodies. Annexin 2 was detected abundant in lipid rafts (>90% of its total expression in cell membranes), also marked as such by the detection of the raft resident proteins flotillin 1 and H-Ras. Conversely, caveolin 1 was detected in the immediately adjacent membrane fractions, and calnexin, an ER protein marker, and β-COP, a Golgi marker, were distributed almost exclusively in denser fractions of the gradient. The Mr of each protein is indicated on the left-hand side. (B) Characteristic protein content distribution of detergent-free cell membrane fractions, showing significantly less protein in the lipid rafts.
Fig. 4. Subcellular distributions of α-, β-, or γ-tubulin indicate actively dividing cells.
ESCs, fixed as described in Methods, were incubated with indicated antibodies and with Hoechst 33258 for chromatin staining. (A) Representative projection confocal images (four focal planes of 0.7µm thickness, at 5 μm from the glass surface in the z-direction) of a single colony show α-tubulin on cytocolic (arrow), mitotic (double arrow), and peripheral (yellow arrow) MTs; last panel contains the merged α-tubulin and Hoechst images. (B) Typical confocal images (projection of four, 0.7 µm sections) of an ESC colony show staining of β3-tubulin in the periphery of the cells (yellow arrow) and in mitotic spindles (double arrow). (C) ESCs stained for α-tubulin (red) and F-actin (green) reveal a strong colocalization correlation (yellow arrows), readily seen in the scatter plot of the last column, which shows signal intensity of each voxel in the plane, as indicated in the label; PCC=0.288 ± 0.05. (D) Representative projection confocal images (four focal planes of 0.5 µm at 5 μm from the glass surface in the Z-direction) of an ESC colony stained with a γ-tubulin antibody decorates paranuclear centrosomes; adjacent staining indicates duplicated centrosomes (asterisks). bars =10 μm.
Annexins
We also identified several annexins - A6 (P14824), A7 (Q07076), and A2 (P07356) - as ESC lipid raft resident proteins (Fig. 2 and Table 1). Annexins are soluble, hydrophilic proteins that may self-associate and bind to negatively charged phospholipids, in a Ca2+-dependent manner, and to F-actin (Raynal and Pollard, 1994). Annexin A2, in particular, most likely provides a structural link between lipid rafts and the cortical cytoskeleton, since treatment with cholesterol-sequestering agents results in its specific release together with the cortical cytoskeletal proteins α-actinin, ezrin, and actin (Harder et al., 1997). Annexin A6 has been shown to promote a cysteine protease-dependent remodelling of the membrane cytoskeleton and may facilitate receptor-mediated budding of clathrin-coated vesicles at the plasma membrane during endocytosis (Smythe et al., 1994). Annexin A7 is involved in mediating the energy-Ca2+-dependent exocytotic membrane fusion, and is pivotal for early embryo development as its deletion in mice leads to embryonic lethality (Gerke and Moss, 2002). We did not detect A5, an annexin that has been suggested as a differentiation marker in rat ST14A progenitor cells (Hoffrogge et al., 2007).
Importantly, Western blotting analysis of annexin 2 in all fractions validated the strong enrichment of the protein in the lipid rafts (Fig. 3). As expected, the lipid raft marker flotillin 1 (Macdonald and Pike, 2005) was concentrated in the same fractions, while H- Ras, another well-established lipid raft marker, was also detected there; caveolin 1, as expected, was faintly detected in fraction 5 of the lipid rafts and more prominent in fractions 6-8. In contrast, calnexin and β-COP, two proteins associated with ER and Golgi, respectively, were clearly segregated away from lipid rafts (Fig. 3A). The high enrichment of annexin 2 in lipid raft was best appreciated when considering the total protein content in the fractions (Fig. 3B). To our knowledge, this is the first time that annexins have been approached by proteomics in lipid rafts.
In interpreting the proteotype of lipid rafts, the overall sparsity of proteins may indicate that the rafts at the time point of our analysis were at the “small” size stage (Gupta et al. 2006; Sezgin et al. 2017; Kusumi et al. 2020). Alternatively, it is possible that activation of receptors and induction of lipid raft merging may be intense in ESCs, in which case clustered rafts may detach from the plasma membrane in a vesicle like form and would not be detected in isolated rafts. This raft-dependent-endocytic pathway has been attracting attention over the past few years, across kingdoms of life and even as a mechanism for entry of viruses, including corona-viruses (Li et al. 2007; Elkin et al. 2016; Wei et al. 2020). Endocytosis requires extensive actin and MT networks, to serve as tracks for intracellular vesicular trafficking. Indeed, use of colchicine and cytochalasin D, which disrupt microtubules and actin filaments, respectively, leads to redistribution of GPCR-Gs-AR in lipid rafts (Head et al., 2006). Hence, our overall results postulate that lipid rafts in ESCs act in close association with the actin and tubulin cytoskeletons to support signal compartmentalization.
Lipid Raft Disruption by Cholesterol Depletion Negates the Mitogenic Effect of Prolactin
Many studies, including ours, have postulated that prolactin, a secreted polypeptide hormone, may act as a mitogen in several cell types, including precursor cells in the adult mouse hippocampus (Walker et al., 2012), embryonic astrocytes (Mangoura et al., 2000), pheochromocytoma and neuroblastoma cells (Cheng et al., 2000), as well as many cancer stem cell types (Rouet et al., 2010). Its growth factor-like actions were further supported by its role in improving the implantation potential in mouse blastocysts (Takeuchi et al., 2017). Its specific receptor, PRLR, an archetype member of the cytokine receptor superfamily and a single-pass transmembrane protein, possesses no enzymatic activity. Thus, it exerts its signalling actions like LIF, through the activation of JAK2, a cytokine-pre associated tyrosine kinase, and the transcription factors STAT1, 3, or 5 (Cheng et al., 2000), and we detected its high expression in ESCs by immunocytochemistry (data not shown). Hence, we used prolactin as mitogen to test whether lipid raft integrity is required for completion of this signalling pathway.
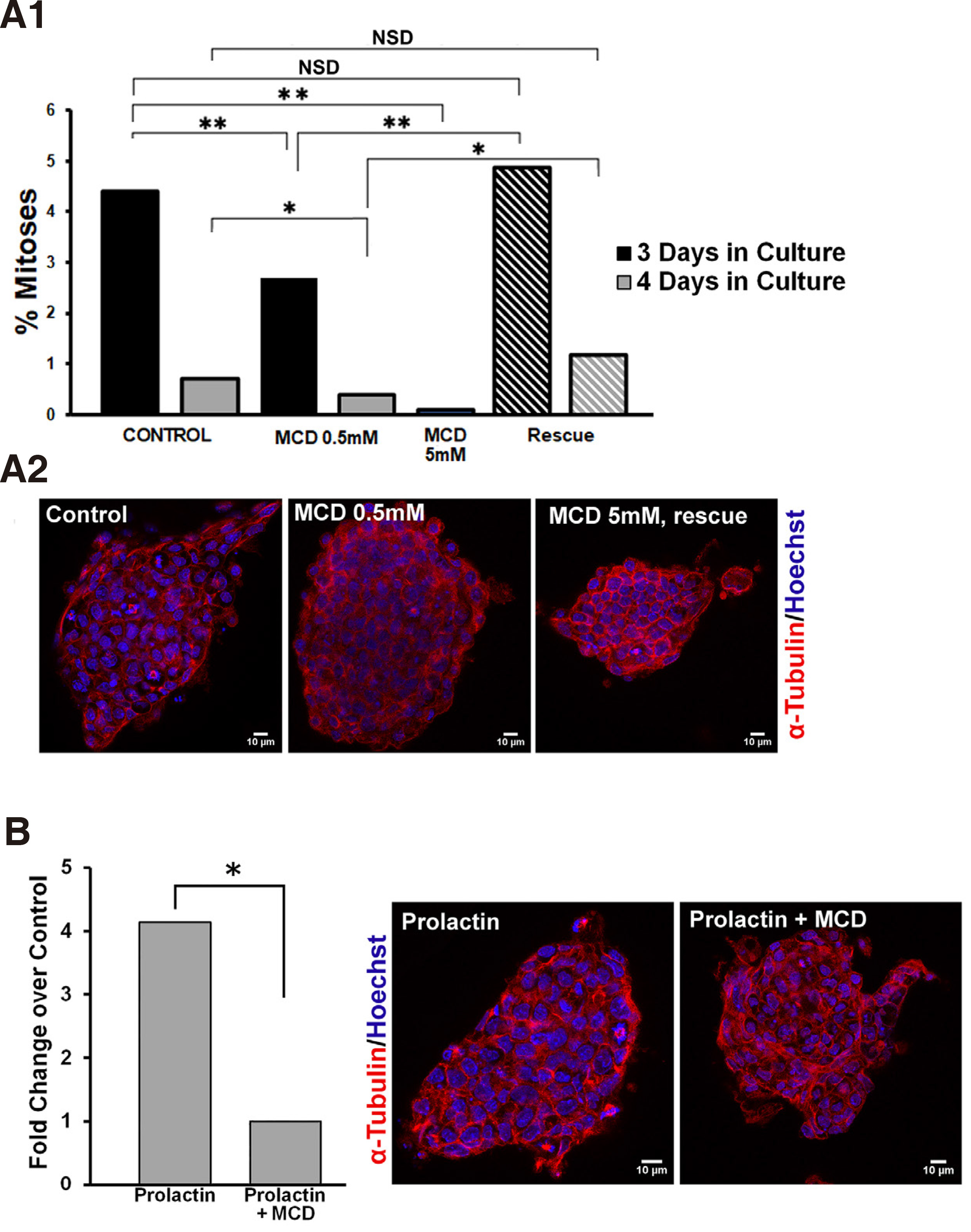
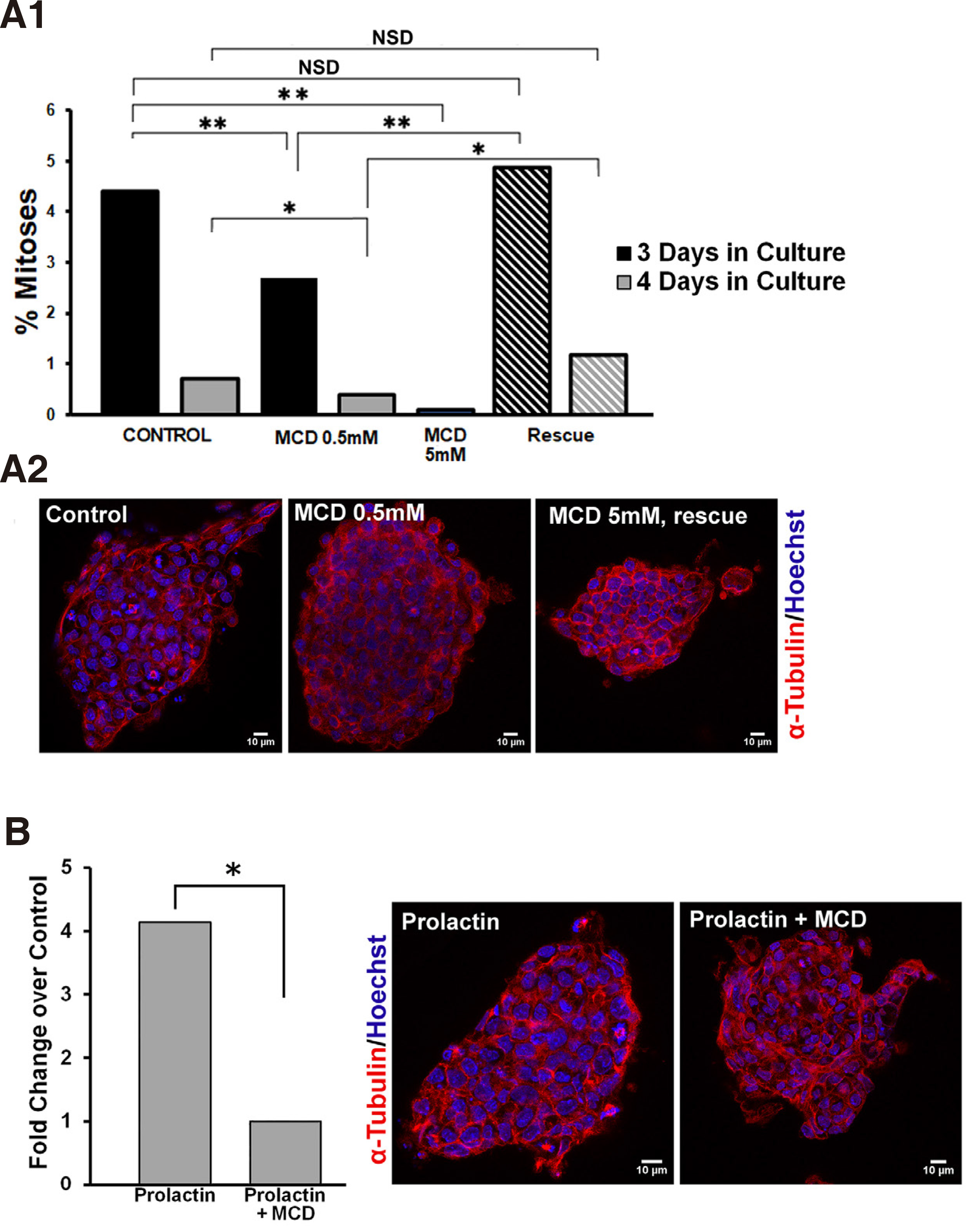
First, we established that lipid raft integrity is necessary for propagation of mitogenic signalling pathways in ESCs. In order to disrupt lipid rafts, we used methyl-beta-cyclodextrin (MCD), a cyclic oligosaccharide that sequesters and thus depletes cells of cholesterol (Asimaki et al. 2011; Mangoura 2016; Karouzaki et al. 2019), for various exposure times and doses, and then recorded the number of mitoses. More specifically, 48 h after plating of ESCs on gelatin coverslips, cultures were treated either with vehicle, or with a low dose of 0.5mM MCD and were left to grow for another 1 or 2 days (Fig. 5A1, black and grey columns, respectively), and were then fixed and processed for mitotic figure analysis as described in Methods. When compared with their control counterparts, namely ESCs grown under identical conditions for 3 or 4 days but without MCD, a significant drop in the percentage of mitotic figures in the total population was evident. Therefore, long treatment with 0.5mM MCD significantly decreased mitotic activities in ESCs. When we used a higher dose of 5mM MCD and an expected >90 % of cellular cholesterol was depleted (Zidovetzki and Levitan, 2007), ESCs not only rarely displayed a mitotic figure, but most appeared to have apoptotic characteristics, such as cytoplasmic membrane blebbing and chromatin fragmentation (data not shown). Further establishing the importance of the plasma membrane cholesterol levels, when ESCs were “rescued” from MCD(i.e. cells were exposed to MCD by 1 h pulses every 24 h),the rates of mitotic figures per 3 or 4 days (stripped columns, respectively) did not differ statistically from controls, as is also clearly seen in typical confocal images in Fig. 5A2.
Fig. 5. Prolactin significantly increases the mitotic index in ESCs and requires intact lipid rafts.
(A1) Lipid raft integrity is required for mitotic expansion of ESCs. ESCs cultured 3 or 4 days in total, black and grey columns, respectively, were treated continuously with vehicle, 0.5mM MCD, or 5mM MCD, or in pulses of 1 hour MCD for the same time periods (hatched columns); upon completion of treatments, the ratio between the number of cells in mitosis and the total number of cells (mitotic index) was calculated in samples immunostained for α-tubulin (red) and co-stained with Hoechst for chromatin (blue). (A2) Representative, single focal planes of samples used in A1. (B) Lipid raft integrity controls the mitogenic effect of prolactin. ESCs were treated after 3 days in culture with 100nM prolactin with or without 0.5 mM MCD, and the resulting mitotic indeces show that the high mitogenic effect of prolactin is abolished by concurrent treatment with MCD. Panels show representative, single focal planes of samples used for these calculations. * p <0.05, ** p ≤0.01, *** p ≤ 0.001, NSD, no significant difference.
As shown in Fig. 5A1, the rate of mitoses under normal culture conditions falls significantly between day 3 and 4 in culture. Therefore, we chose to treat cells with prolactin (100nM) on the 3rd day and assess numbers of mitotic figures in the presence or absence of 0.5mM MCD. Prolactin acted as a strong mitogen in ESCs, with a significant 4-fold rise in the numbers of mitoses in the total cell populations (Fig. 5B). This mitogenic effect was curtailed significantly by MCD, also clearly visible in confocal images of cells stained for alpha tubulin and Hoechst. Therefore, while the association of the prolactin receptor subunits with the lipid rafts needs to be further investigated, similarly, our results suggest that lipid rafts are signalling platforms for the prolactin-induced mitogenic pathway in ESCs. Our studies highlight the critical role of well-organized lipid rafts in the intracellular transduction of signals from the PRLR, a receptor that is widely expressed in stem cells and which gains multifold expression prior to blastulation of human embryos (Ezoe et al., 2021). Moreover, blastocyst outgrowth is also significantly increased in human embryos cultured in the presence of PRL, further strengthening the suggestion that manipulation of lipid rafts in stem cells may be a target for controlling their expansion, a field of considerable interest for cancer stem cells (Rouet et al., 2010)).
Concluding Remark
We have further defined and expanded the mouse E14 stem cell proteome and have highlighted in particular its close association with the major cytoskeleton assemblies of actins and tubulins. Apparently, ESCs have strikingly developed cytoskeletons, unexpected for non-polarized cells, giving rise to speculation that the close association between the signalling platforms of the lipid rafts and the actin and tubulin systems may prevent cell polarization. As ESCs grow exclusively in situ as multi-cell colonies with their cell motion practically restricted to cytokinesis, this complex lipid raft- cytoskeleton assembly may also regulate lipid raft-dependent signalling for self-renewal and growth, i.e. for symmetric cell division.
Materials and Methods
Antibodies
Goat polyclonal antibody to calnexin was purchased from Santa Cruz Biotechnology (SCBT). Mouse monoclonal antibodies to actin, coatomer protein beta (β-COP), and to α- and β-tubulin from Sigma-Aldrich; to tubulin β3 (TUJ1) from Covance, to γ-tubulin from Abcam, and to flotilin-1 from SCBT. Rabbit polyclonal antibodies to annexin 2 and a rat monoclonal antibody to H-Ras were also from SCBT.
Chemicals
Methyl-beta-cyclodextrin (MCD) was purchased from Sigma-Aldrich, and prolactin was kindly provided by the National Hormone and Pituitary Program, National Institutes of Health. The source of all chemicals and reagents used is indicated when first described; standard chemicals were from Sigma.
ES Cell culture
The E14 ESC line (Chambers et al., 2003) was maintained on 0.1% gelatin-coated dishes without a feeder layer of fibroblasts (Bibel et al., 2004) in high-glucose (10%) DMEM, supplemented with 15% stem cell-certified fetal bovine serum, FBS (Biowest), 6mM L-glutamine, non-essential amino acids (Invitrogen), 1000 units/ml LIF (Millipore), and 0.1mM β-Mercaptoethanol (Acros Organics), at 37˚C and 10% CO2. The W4 cell line (a generous gift from Dr. A. Klinakis, BRFAA), used to verify lipid rafts protein expression, was cultured under identical conditions.
ES Cell culture treatments
Treatments on ESCs were done routinely after two days in culture, using 0.5mM or 5mM of the cholesterol-sequestering agent Methyl-β-cyclodextrin (MCD) and/or 100nM Prolactin in the presence of 3% FBS and LIF.
Membrane subfractionation
Lipid rafts were isolated using a detergent-free method that preserves the native composition of these lipid-protein microdomains, as previously reported (Macdonald and Pike 2005; Asimaki et al. 2011; Karouzaki et al. 2019). In brief, 4x106 cells were resuspended in base buffer consisting of 20 mM Tris-HCl pH 7.8, 250 mM sucrose, 1 mM calcium chloride, 1 mM magnesium chloride, and protease and phosphatase inhibitors and then lysed by repeated passage through a 23 G needle. Lysates were centrifuged at 1000 x g for 10 min, and the resulting postnuclear supernatant was collected and transferred to a separate tube. An equal volume of base buffer containing 50% OptiPrep was added, and all were placed in the bottom of a 12 ml centrifuge tube. A 10 ml gradient of 0% to 20% OptiPrep in base buffer was poured on top and gradients were centrifuged for 90 min at 52,000 x g using a TH-641 rotor in a Sorval Discovery 90 ultracentrifuge. Gradients were fractionated into fourteen 0.7 ml fractions. As the total protein content was low (Asimaki et al. 2011; Karouzaki et al. 2019), the first five fractions, representing lipid rafts, were pooled for proteomics analysis, while for Western blotting analysis they were run individually. Fractions or lysates were boiled in Laemmli buffer and the distribution of the various proteins was assessed with 1DE and Western blotting or 2DE and MS-MS.
Two-dimension electrophoresis (DE) of gels and peptide mass fingerprint (PMF)
2D gel electrophoresis was performed as previously described (Anagnostopoulos et al., 2011). Briefly, 1 mg of protein was applied on 18cm immobilized pH 3-10 non-linear gradient strips (Bio-Rad) and isoelectric focusing was carried out with at 3 V/min for 18 h; the second-dimensional separation was performed in 12% SDS-polyacrylamide gels at well-defined conditions. Proteins were visualized with colloidal Coomassie Brilliant Blue and scanned with a GS-800 densitometer.
Peptide analysis and protein identification were performed as described (Berndt et al., 1999). Spots were automatically detected by Melanie 4.02 software (GeneBio) on the Coomassie-stained gels, excised by the Proteiner SPII (Bruker Daltonics), destained with 30% acetonitrile in 50 mM ammonium bicarbonate and dried in a speed vacuum concentrator. Each dried gel piece was rehydrated with 5μl of 1 mM ammonium bicarbonate containing 50 ng trypsin, overnight at room temperature. Twenty microliters of 50% acetonitrile - 0.1% trifluoroacetic acid were added for 15 min with constant shaking. The resulting peptide mixture (1 μl) was simultaneously applied with 1 μl of matrix solution, consisting of 0.8% α-cyano-4-hydroxycinnamic, standard peptides des-Arg-bradykinin (904.46 kD), and adrenocorticotropic hormone fragment 18-39 (2465.19 kD). Samples were analyzed for PMF with Matrix-Assisted Laser Desorption-Mass Spectrometry (MALDI-MS) in a time-of-flight mass spectrometer (Ultraflex II). Laser shots (n = 400) at intensity between 40% and 60% were collected and summarized, and peak lists were created using the Bruker FlexControl v2.2 and Flexanalysis v2.2 software. Each spectrum was interpreted with Mascot Software v2.0 (Matrix Sciences). For peptide identification, the monoisotopic masses were used and a mass tolerance of 0.0025% (25ppm) was allowed. All extraneous peaks, such as trypsin autodigests, matrix, and keratin peaks, were not considered for the protein search. Peptide masses were compared with the theoretical peptide masses of all available proteins from rodents in the SWISS-PROT and TREmBL databases, which are updated bimonthly. The probability score identified by the software was used as the criterion for identification.
Western blotting (WB)
WB analysis was essentially performed as previously described (e.g. Karouzaki et al., 2019). Lipid raft fractions were analyzed as equal volumes (50 µl) of OptiPrep fractions in Laemmli buffer per well of a polyacrylamide gel by electrophoresis (SDS-PAGE), after which proteins were transferred onto nitrocellulose membranes and probed with primary antibodies. Immunoreactivity was visualized by incubation with the appropriate species-specific antibody, conjugated to horseradish peroxidase (HRP) or alkaline phosphatase (AP), and ECL chemiluminescence or AP substrate colorimetric reaction, respectively. Exposed films or stained membranes were then scanned and exported as TIFF files. All experiments were repeated at least four times with similar results.
Immunocytochemistry
For immunocytodetection of proteins, cells were treated with the protein crosslinker disuccinimidyl propionate (DSP), in order to best preserve cytoskeletal assemblies (Leondaritis et al. 2009; Peta et al. 2020), extracted with 0.1% Triton, and then fixed with 4% paraformaldehyde (PFA). After blocking of non-specific sites with serum from the appropriate species, cells were first incubated with primary antibodies and then with the appropriate secondary antibodies conjugated to rhodamine (red), while chromatin was stained with Hoechst 33258 (blue) (Pharmingen). F-actin was visualized with Oregon Green 488 phalloidin (ThermoFisher) or Alexa Fluor 633 phalloidin (Invitrogen) staining. Stained cells were imaged with a Leica TCS SPT laser inverted scanning confocal microscope, equipped with two ion lasers through a 63X HC PC APO CS objectives and Tandem Scanner (Leica Microsystems, Biological imaging facility, BRFAA), and z-stacks with 0.34µm-thick z optical sections. All Images were analyzed using LAS-AF software, deconvolved using Huygens Essential (SVI) and saved in a TIFF format.
Quantification of mitotic cells
The analysis of the mitotic cells was performed with the Cell Counter plugin of the ImageJ software. The area of the nucleus was defined from Hoechst fluorescence, and the mitotic cells from Hoechst fluorescence and β-tubulin immunostaining of confocal images, captured in z-stacks with 0.34 µm-thick z optical sections. All mitotic cells (in prophase, metaphase, anaphase or telophase) and nuclear foci were marked with a coloured square, given a unique number, and added to a tally sheet; results were exported as an Excel file for statistical analysis. The total percentage of mitoses that were calculated as mitotic figures over total numbers of nuclei from 2-3000 cells for each condition (Fig. 5).
Colocalization Analysis
Colocalization analysis of α-tubulin and F-actin was performed with the Volocity® 6.1.2 software, using the Costes Pearson Correlation Coefficient (PCC) algorithm as described in (Peta et al., 2020). Regions of interest (ROI) were drawn manually around the cells (100 cells from 3 different experiments) and the extent of overlap between fluorophores were calculated within a range of 1, if a perfect positive correlation, to -1, if a perfect but inverse correlation; 0 represents a random distribution. PCC means were automatically calculated from the generated scattered plots and exported for every single plane in an excel file.
Semi-quantitative PCR
SemiQ-PCR was performed as previously described (Mangoura et al. 2006; McLaughlin et al. 2006). Briefly, total RNA isolated from embryonic stem cell cultures, using the Trizol reagent (Ambion), was reverse transcribed (1 µg per reaction) with SuperScript II reverse transcriptase (Invitrogen), and 1µL of the formed cDNA was amplified with Taq DNA Polymerase (New England Biolabs), in the presence of gene-specific primers for: Nanog-P1, 5’ GCGGACTGTGTGTTCTCTCAGGC 3’; Nanog-P2, 5’ TTCCAGATCCGTTCACCAGATAG 3’; Oct4-P1, 5’ GGCGTTCGCTTTGGAAAGGTGTTC 3’; Oct4-P2, 5’ CTCGAACCACATCCTTCTCT 3’; and, used as control, β-actin-P1, 5’ TGACGGGGTCACCCACACTGTGCCCATCTA 3’; β-actin-P2, 5’ CTAGAAGCAGCGGTGGAGGATGGATGGA GGG 3’. Amplification conditions, for Nanog and Oct4, were an initial denaturation step of 5 min at 94°C followed by 33-40 cycles (linear region) at 94°C for 1 min, at 60° C for 30 sec, at 72° C for 30 sec, followed by 10 min at 72°C. For β-actin, the corresponding amplification steps were: 94° C, 5 min; 94° C, 1 min; 56° C, 30 sec; 72° C, 1 min; final extension 72° C for 5 min. PCR products, separated in appropriate for each product percentage agarose gels, were stained with ethidium bromide and photographed under UV light using the Dolphin-Doc Pro system.
Statistics
All experiments were performed three to ten times with similar results, and numerical data were analyzed by ANOVA, using the SPSS software (IBM) and a set statistical significance level (p) of 0.05. Graphs were generated with the same software, and images were organized using Adobe Photoshop.
Acknowledgements
We acknowledge the assistance of Dr. P. Kouklis (University of Ioannina) with stem cell cultures during the early phases of the project.
Abbreviations
ESCs, Embryonic stem cells ; MCD, Methyl-β-Cyclodextrin ; MT, Microtubules ; PRL, Prolactin ;References
Allen J. A., Yu J. Z., Dave R. H., Bhatnagar A., Roth B. L., Rasenick M. M. (2009). Caveolin-1 and Lipid Microdomains Regulate G s Trafficking and Attenuate G s /Adenylyl Cyclase Signaling . Molecular Pharmacology 76: 1082-1093.
Anagnostopoulos A. K., Dimas K. S., Papathanassiou C., Braoudaki M., Anastasiadou E., Vougas K., Karamolegou K., Kontos H., Prodromou N., Tzortzatou-Stathopoulou F., Tsangaris G. T. (2011). Proteomics Studies of Childhood Pilocytic Astrocytoma. Journal of Proteome Research 10: 2555-2565.
Androutsellis-Theotokis A., Walbridge S., Park D. M., Lonser R. R., McKay R. D. G. (2010). Cholera Toxin Regulates a Signaling Pathway Critical for the Expansion of Neural Stem Cell Cultures from the Fetal and Adult Rodent Brains. PLoS ONE 5: e10841.
Araujo A. R., Gelens L., Sheriff R. S.M., Santos S. D.M. (2016). Positive Feedback Keeps Duration of Mitosis Temporally Insulated from Upstream Cell-Cycle Events. Molecular Cell 64: 362-375.
Asimaki O., Leondaritis G., Lois G., Sakellaridis N., Mangoura D. (2011). Cannabinoid 1 receptor-dependent transactivation of fibroblast growth factor receptor 1 emanates from lipid rafts and amplifies extracellular signal-regulated kinase 1/2 activation in embryonic cortical neurons. Journal of Neurochemistry 116: 866-873.
Asimaki O., Mangoura D. (2011). Cannabinoid receptor 1 induces a biphasic ERK activation via multiprotein signaling complex formation of proximal kinases PKCɛ, Src, and Fyn in primary neurons. Neurochemistry International 58: 135-144.
Ballestrem C., Wehrle-Haller B., Hinz B., Imhof B. A. (2000). Actin-dependent Lamellipodia Formation and Microtubule-dependent Tail Retraction Control-directed Cell Migration. Molecular Biology of the Cell 11: 2999-3012.
Banani S. F., Lee H. O., Hyman A. A., Rosen M. K. (2017). Biomolecular condensates: organizers of cellular biochemistry. Nature Reviews Molecular Cell Biology 18: 285-298.
Berndt P., Hobohm U., Langen H. (1999). Reliable automatic protein identification from matrix-assisted laser desorption/ionization mass spectrometric peptide fingerprints. Electrophoresis 20: 3521-3526.
Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., Jaenisch R., Wagschal A., Feil R., Schreiber S. L., Lander E. S. (2006). A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell 125: 315-326.
Bibel M., Richter J., Schrenk K., Tucker K. L., Staiger V., Korte M., Goetz M., Barde Y.A. (2004). Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nature Neuroscience 7: 1003-1009.
Bittermann E., Abdelhamed Z., Liegel R. P., Menke C., Timms A., Beier D. R., Stottmann R. W. (2019). Differential requirements of tubulin genes in mammalian forebrain development. PLOS Genetics 15: e1008243.
Breuss M. W., Leca I., Gstrein T., Hansen A. H., Keays D. A. (2017). Tubulins and brain development – The origins of functional specification. Molecular and Cellular Neuroscience 84: 58-67.
Brook F. A., Gardner R. L. (1997). The origin and efficient derivation of embryonic stem cells in the mouse. Proceedings of the National Academy of Sciences 94: 5709-5712.
Brouhard G. J., Rice L. M. (2018). Microtubule dynamics: an interplay of biochemistry and mechanics. Nature Reviews Molecular Cell Biology 19: 451-463.
Burdon Tom, Smith Austin, Savatier Pierre, (2002). Signalling, cell cycle and pluripotency in embryonic stem cells. Trends in Cell Biology 12: 432-438.
Case L. B., Zhang X., Ditlev J. A., Rosen M. K., (2019). Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 363: 1093-1097.
Chaaban S., Brouhard G. J. (2017). A microtubule bestiary: structural diversity in tubulin polymers. Molecular Biology of the Cell 28: 2924-2931.
Chacon J., Rogers C. D. (2019). Early expression of Tubulin Beta-III in avian cranial neural crest cells. Gene Expression Patterns 34: 119067.
Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003). Functional Expression Cloning of Nanog, a Pluripotency Sustaining Factor in Embryonic Stem Cells. Cell 113: 643-655.
Chen H., Guo R., Zhang Q., Guo H., Yang M., Wu Z., Gao S., Liu L., Chen L. (2015). Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells. Proceedings of the National Academy of Sciences 112: E5936-E5943.
Chen K., Williams K. J. (2013). Molecular Mediators for Raft-dependent Endocytosis of Syndecan-1, a Highly Conserved, Multifunctional Receptor. Journal of Biological Chemistry 288: 13988-13999.
Chen Y., Veracini L., Benistant C., Jacobson K. (2009). The transmembrane protein CBP plays a role in transiently anchoring small clusters of Thy-1, a GPI-anchored protein, to the cytoskeleton. Journal of Cell Science 122: 3966-3972.
Cheng Y., Zhizhin I., Perlman R. L., Mangoura D. (2000). Prolactin-induced Cell Proliferation in PC12 Cells Depends on JNK but Not ERK Activation. Journal of Biological Chemistry 275: 23326-23332.
Chichili G. R., Rodgers W. (2007). Clustering of Membrane Raft Proteins by the Actin Cytoskeleton. Journal of Biological Chemistry 282: 36682-36691.
Dinic J., Riehl A., Adler J., Parmryd I. (2015). The T cell receptor resides in ordered plasma membrane nanodomains that aggregate upon patching of the receptor. Scientific Reports 5: 10082.
Dremina E. S., Sharov V. S., Schöneich C. (2005). Protein tyrosine nitration in rat brain is associated with raft proteins, flotillin-1 and α-tubulin: effect of biological aging. Journal of Neurochemistry 93: 1262-1271.
Elkin S. R., Lakoduk A. M., Schmid S. L. (2016). Endocytic pathways and endosomal trafficking: a primer. Wiener Medizinische Wochenschrift 166: 196-204.
Ezoe K., Miki T., Ohata K., Fujiwara N., Yabuuchi A., Kobayashi T., Kato K. (2021). Prolactin receptor expression and its role in trophoblast outgrowth in human embryos. Reproductive BioMedicine Online 42: 699-707.
Ferreira L. T., Figueiredo A. C., Orr B., Lopes D., Maiato H., (2018). Dissecting the role of the tubulin code in mitosis. In Mitosis and Meiosis Part A. Elsevier.
Gerke V., Moss S. E. (2002). Annexins: From Structure to Function. Physiological Reviews 82: 331-371.
Goswami D., Gowrishankar K., Bilgrami S., Ghosh S., Raghupathy R., Chadda R., Vishwakarma R., Rao M., Mayor S. (2008). Nanoclusters of GPI-Anchored Proteins Are Formed by Cortical Actin-Driven Activity. Cell 135: 1085-1097.
Gupta N., Wollscheid B., Watts J. D., Scheer B., Aebersold R., DeFranco A. L. (2006). Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor–mediated lipid raft dynamics. Nature Immunology 7: 625-633.
Harayama T., Riezman H. (2018). Understanding the diversity of membrane lipid composition. Nature Reviews Molecular Cell Biology 19: 281-296.
Harder T., Kellner R., Parton R. G., Gruenberg J. (1997). Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol.. Molecular Biology of the Cell 8: 533-545.
Hausrat T. J., Radwitz J., Lombino F. L., Breiden P., Kneussel M. (2021). Alpha‐ and beta‐tubulin isotypes are differentially expressed during brain development. Developmental Neurobiology 81: 333-350.
Head B. P., Patel H. H., Roth D. M., Murray F., Swaney J. S., Niesman I. R., Farquhar M. G., Insel P. A. (2006). Microtubules and Actin Microfilaments Regulate Lipid Raft/Caveolae Localization of Adenylyl Cyclase Signaling Components. Journal of Biological Chemistry 281: 26391-26399.
Hoffrogge R., Beyer S., Hübner R., Mikkat S., Mix E., Scharf C., Schmitz U., Pauleweit S., Berth M., Zubrzycki I. Z., Christoph H., Pahnke J., Wolkenhauer O., Uhrmacher A., Völker U., Rolfs A. (2007). 2-DE profiling of GDNF overexpression-related proteome changes in differentiating ST14A rat progenitor cells. PROTEOMICS 7: 33-46.
Huang G., Ye S., Zhou X., Liu D., Ying Q.L. (2015). Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network. Cellular and Molecular Life Sciences 72: 1741-1757.
Huang W. Y. C., Alvarez S., Kondo Y., Lee Y. K., Chung J. K., Lam H. Y. M., Biswas K. H., Kuriyan J., Groves J. T. (2019). A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363: 1098-1103.
Intoh A., Kurisaki A., Yamanaka Y., Hirano H., Fukuda H., Sugino H., Asashima M. (2009). Proteomic analysis of membrane proteins expressed specifically in pluripotent murine embryonic stem cells. PROTEOMICS 9: 126-137.
Janke C., Magiera M. M. (2020). The tubulin code and its role in controlling microtubule properties and functions. Nature Reviews Molecular Cell Biology 21: 307-326.
Jaqaman K., Ditlev J. A. (2021). Biomolecular condensates in membrane receptor signaling. Current Opinion in Cell Biology 69: 48-54.
Karouzaki S., Peta C., Tsirimonaki E., Mangoura D. (2019). PKCε-dependent H-Ras activation encompasses the recruitment of the RasGEF SOS1 and of the RasGAP neurofibromin in the lipid rafts of embryonic neurons. Neurochemistry International 131: 104582.
Kawabe J., Okumura S., Nathanson M. A., Hasebe N., Ishikawa Y. (2006). Caveolin regulates microtubule polymerization in the vascular smooth muscle cells. Biochemical and Biophysical Research Communications 342: 164-169.
Kodama A., Karakesisoglou I., Wong E., Vaezi A., Fuchs E. (2003). ACF7. Cell 115: 343-354.
Kusumi A., Fujiwara T. K., Morone N., Yoshida K. J., Chadda R., Xie M., Kasai R. S., Suzuki K. G.N. (2012). Membrane mechanisms for signal transduction: The coupling of the meso-scale raft domains to membrane-skeleton-induced compartments and dynamic protein complexes. Seminars in Cell & Developmental Biology 23: 126-144.
Kusumi A., Fujiwara T. K., Tsunoyama T. A., Kasai R. S., Liu A.A., Hirosawa K. M., Kinoshita M., Matsumori N., Komura N., Ando H., Suzuki K. G. N. (2020). Defining raft domains in the plasma membrane. Traffic 21: 106-137.
Kusumi A., Suzuki K. (2005). Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1746: 234-251.
Leandro-García L. J., Leskelä S., Landa I., Montero-Conde C., López-Jiménez E., Letón R., Cascón A., Robledo M., Rodríguez-Antona C. (2010). Tumoral and tissue-specific expression of the major human β-tubulin isotypes. Cytoskeleton 67: 214-223.
Lee M. Y., Ryu J. M., Lee S. H., Park J. H., Han H. J. (2010). Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. Journal of Lipid Research 51: 2082-2089.
Leondaritis G., Petrikkos L., Mangoura D. (2009). Regulation of the Ras-GTPase activating protein neurofibromin by C-tail phosphorylation: implications for protein kinase C/Ras/extracellular signal-regulated kinase 1/2 pathway signaling and neuronal differentiation. Journal of Neurochemistry 109: 573-583.
Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. (2007). Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes and Infection 9: 96-102.
Li N., Shaw A. R. E., Zhang N., Mak A., Li L. (2004). Lipid raft proteomics: Analysis of in-solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins by liquid chromatography-matrix-assisted laser desorption/ionization tandem mass spectrometry. PROTEOMICS 4: 3156-3166.
Loh Y.H., Wu Q., Chew J.L., Vega V. B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J., Wong K.Y., Sung K. W., Lee C. W. H., Zhao X.D., Chiu K.P., Lipovich L., Kuznetsov V. A., Robson P., Stanton L. W., Wei C.L., Ruan Y., Lim B., Ng H.H. (2006). The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genetics 38: 431-440.
Lorent J. H., Diaz-Rohrer B., Lin X., Spring K., Gorfe A. A., Levental K. R., Levental I. (2017). Structural determinants and functional consequences of protein affinity for membrane rafts. Nature Communications 8: 1219.
Ludueña R. F., Banerjee A., (2008). The Isotypes of Tubulin. In The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. (Ed. Fojo T., ) Humana Press.
Ma X., Chen H., Chen L. (2016). A dual role of Erk signaling in embryonic stem cells. Experimental Hematology 44: 151-156.
Macdonald J. L., Pike L. J. (2005). A simplified method for the preparation of detergent-free lipid rafts. Journal of Lipid Research 46: 1061-1067.
Mangoura D., Asimaki O., Tsirimonaki E., Sakellaridis N., (2016). Role of Lipid Rafts and the Underlying Filamentous-Actin Cytoskeleton in Cannabinoid Receptor 1 Signaling. In Neuropathology of Drug Addictions and Substance Misuse. (Ed. Preedy V. R., ) Academic Press.
Mangoura D., Pelletiere C., Leung S., Sakellaridis N., Wang D. X. (2000). Prolactin concurrently activates Src‐PLD and JAK/Stat signaling pathways to induce proliferation while promoting differentiation in embryonic astrocytes. International Journal of Developmental Neuroscience 18: 693-704.
Mangoura D., Sun Y., Li C., Singh D., Gutmann D. H., Flores A., Ahmed M., Vallianatos G. (2006). Phosphorylation of neurofibromin by PKC is a possible molecular switch in EGF receptor signaling in neural cells. Oncogene 25: 735-745.
Martin G. R. (1980). Teratocarcinomas and Mammalian Embryogenesis. Science 209: 768-776.
McLaughlin D., Tsirimonaki E., Vallianatos G., Sakellaridis N., Chatzistamatiou T., Stavropoulos-Gioka C., Tsezou A., Messinis I., Mangoura D. (2006). Stable expression of a neuronal dopaminergic progenitor phenotype in cell lines derived from human amniotic fluid cells. Journal of Neuroscience Research 83: 1190-1200.
Mitchison T., Kirschner M. (1984). Dynamic instability of microtubule growth. Nature 312: 237-242.
Montecinos-Franjola F., Chaturvedi S. K., Schuck P., Sackett D. L. (2019). All tubulins are not alike: Heterodimer dissociation differs among different biological sources. Journal of Biological Chemistry 294: 10315-10324.
Morone N., Fujiwara T., Murase K., Kasai R. S., Ike H., Yuasa S., Usukura J., Kusumi A. (2006). Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. Journal of Cell Biology 174: 851-862.
Morris R., Cox H., Mombelli E., Quinn P. J. (2004). Rafts, Little Caves and Large Potholes: How Lipid Structure Interacts with Membrane Proteins to Create Functionally Diverse Membrane Environments. In Membrane Dynamics and Domains. (Ed. Quinn P. J.) Springer US, Boston, MA.
Nagano K., Taoka M., Yamauchi Y., Itagaki C., Shinkawa T., Nunomura K., Okamura N., Takahashi N., Izumi T., Isobe T. (2005). Large-scale identification of proteins expressed in mouse embryonic stem cells. PROTEOMICS 5: 1346-1361.
Nakamura K., Salomonis N., Tomoda K., Yamanaka S., Conklin B. R. (2009). Gi-Coupled GPCR Signaling Controls the Formation and Organization of Human Pluripotent Colonies. PLoS ONE 4: e7780.
Perrin B. J., Ervasti J. M. (2010). The actin gene family: Function follows isoform. Cytoskeleton 67: 630-634.
Persaud-Sawin D.A., Lightcap S., Harry G. J. (2009). Isolation of rafts from mouse brain tissue by a detergent-free method. Journal of Lipid Research 50: 759-767.
Peta C., Tsirimonaki E., Samouil D., Georgiadou K., Mangoura D. (2020). Nuclear Isoforms of Neurofibromin Are Required for Proper Spindle Organization and Chromosome Segregation. Cells 9: 2348.
Portran D., Schaedel L., Xu Z., Théry M., Nachury M. V. (2017). Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nature Cell Biology 19: 391-398.
Puri C., Tosoni D., Comai R., Rabellino A., Segat D., Caneva F., Luzzi P., Di Fiore P. P., Tacchetti C. (2005). Relationships between EGFR Signaling–competent and Endocytosis-competent Membrane Microdomains. Molecular Biology of the Cell 16: 2704-2718.
Raghupathy R., Anilkumar A. A., Polley A., Singh P. P., Yadav M., Johnson C., Suryawanshi S., Saikam V., Sawant S. D., Panda A., Guo Z., Vishwakarma R. A., Rao M., Mayor S. (2015). Transbilayer Lipid Interactions Mediate Nanoclustering of Lipid-Anchored Proteins. Cell 161: 581-594.
Raynal P., Pollard H. B., (1994). Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes 1197: 63-93.
Ren W., Jhala U. S., Du K. (2013). Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte 2: 17-27.
Rollason R., Korolchuk V., Hamilton C., Jepson M., Banting G. (2009). A CD317/tetherin–RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. Journal of Cell Biology 184: 721-736.
Rouet V., Bogorad R. L., Kayser C., Kessal K., Genestie C., Bardier A., Grattan D. R., Kelder B., Kopchick J. J., Kelly P. A., Goffin V. (2010). Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proceedings of the National Academy of Sciences 107: 15199-15204.
Schmidt L., Wain K. E., Hajek C., Estrada-Veras J. I., Guillen Sacoto M. J., Wentzensen I. M., Malhotra A., Clause A., Perry D., Moreno-De-Luca A., Bell M. (2021). Expanding the Phenotype of TUBB2A-Related Tubulinopathy: Three Cases of a Novel, Heterozygous TUBB2A Pathogenic Variant p.Gly98Arg. Molecular Syndromology 12: 33-40.
Schoenenberger C.A., Mannherz H. G., Jockusch B. M. (2011). Actin: From structural plasticity to functional diversity. European Journal of Cell Biology 90: 797-804.
Schröter J., Döring J. H., Garbade S. F., Hoffmann G. F., Kölker S., Ries M., Syrbe S. (2021). Cross-sectional quantitative analysis of the natural history of TUBA1A and TUBB2B tubulinopathies. Genetics in Medicine 23: 516-523.
Schuster B. S., Regy R. M., Dolan E. M., Kanchi Ranganath A., Jovic N., Khare S. D., Shi Z., Mittal J. (2021). Biomolecular Condensates: Sequence Determinants of Phase Separation, Microstructural Organization, Enzymatic Activity, and Material Properties. The Journal of Physical Chemistry B 125: 3441-3451.
Sekino Y., Han X., Babasaki T., Miyamoto S., Kitano H., Kobayashi G., Goto K., Inoue S., Hayashi T., Teishima J., Sakamoto N., Sentani K., Oue N., Yasui W., Matsubara A. (2020). TUBB3 Is Associated with High-Grade Histology, Poor Prognosis, p53 Expression, and Cancer Stem Cell Markers in Clear Cell Renal Cell Carcinoma. Oncology 98: 689-698.
Sezgin E., Levental I., Mayor S., Eggeling C. (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature Reviews Molecular Cell Biology 18: 361-374.
Sferra A., Fattori F., Rizza T., Flex E., Bellacchio E., Bruselles A., Petrini S., Cecchetti S., Teson M., Restaldi F., Ciolfi A., Santorelli F. M., Zanni G., Barresi S., Castiglioni C., Tartaglia M., Bertini E. (2018). Defective kinesin binding of TUBB2A causes progressive spastic ataxia syndrome resembling sacsinopathy. Human Molecular Genetics 27: 1892-1904.
Shin Y., Brangwynne C. P. (2017). Liquid phase condensation in cell physiology and disease. Science 357: eaaf4382.
Simons K., Gerl M. J. (2010). Revitalizing membrane rafts: new tools and insights. Nature Reviews Molecular Cell Biology 11: 688-699.
Simons K., Sampaio J. L. (2011). Membrane Organization and Lipid Rafts. Cold Spring Harbor Perspectives in Biology 3: a004697-a004697.
Smythe E., Smith P.D., Jacob S.M., Theobald J., Moss S.E. (1994). Endocytosis occurs independently of annexin VI in human A431 cells. Journal of Cell Biology 124: 301-306.
Sobierajska K., Wieczorek K., Ciszewski W. M., Sacewicz-Hofman I., Wawro M. E., Wiktorska M., Boncela J., Papiewska-Pajak I., Kwasniak P., Wyroba E., Cierniewski C. S., Niewiarowska J. (2016). β-III tubulin modulates the behavior of Snail overexpressed during the epithelial-to-mesenchymal transition in colon cancer cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863: 2221-2233.
Takeuchi M., Seki M., Furukawa E., Takahashi A., Saito K., Kobayashi M., Ezoe K., Fukui E., Yoshizawa M., Matsumoto H. (2017). Improvement of implantation potential in mouse blastocysts derived from IVF by combined treatment with prolactin, epidermal growth factor and 4-hydroxyestradiol. MHR: Basic science of reproductive medicine 23: 557-570.
Taylor K. D., Pikó L. (1990). Quantitative changes in cytoskeletal β- and γ-actin mRNAS and apparent absence of sarcomeric actin gene transcripts in early mouse embryos. Molecular Reproduction and Development 26: 111-121.
Thomson J. A., Marshall V. S. (1998). 4 Primate Embryonic Stem Cells. In Current Topics in Developmental Biology Volume 38. Elsevier.
Tsvetkov A. S., Samsonov A., Akhmanova A., Galjart N., Popov S. V. (2007). Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motility and the Cytoskeleton 64: 519-530.
Walker T. L., Vukovic J., Koudijs M. M., Blackmore D. G., Mackay E. W., Sykes A. M., Overall R. W., Hamlin A. S., Bartlett P. F. (2012). Prolactin Stimulates Precursor Cells in the Adult Mouse Hippocampus. PLoS ONE 7: e44371.
Wang D., Gao L. (2005). Proteomic analysis of neural differentiation of mouse embryonic stem cells. PROTEOMICS 5: 4414-4426.
Wei X., She G., Wu T., Xue C., Cao Y. (2020). PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Veterinary Research 51: 10.
Xu X., Warrington A. E., Wright B. R., Bieber A. J., Van Keulen V., Pease L. R., Rodriguez M. (2011). A human IgM signals axon outgrowth: coupling lipid raft to microtubules. Journal of Neurochemistry 119: 100-112.
Zidovetzki R., Levitan I. (2007). Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochimica et Biophysica Acta (BBA) - Biomembranes 1768: 1311-1324.