Int. J. Dev. Biol. 67: 101 - 108 (2023)
Valproic acid effects on human adipose-derived stem cell differentiation into oligodendrocytes and improved remyelination in a mouse model of Multiple Sclerosis
Original Article | Published: 31 October 2023
Abstract
Valproic acid (VPA), a neuroprotective agent and inhibitor of GSK3-β, along with human Adipose-Derived Stem Cells (hADSCs) have been proposed to be potential therapeutic agents for neurodegenerative disorders. In the present study, we have assessed the effects of VPA alone or in combination with hADSCs on oligodendrocyte differentiation, remyelination, and functional recovery in a mouse model of Multiple Sclerosis (MS). These MS-model mice were randomly divided into cuprizone, sham, VPA, hADSC, and VPA/hADSC groups, with 10 mice considered a control group (healthy mice). The hanging wire test was used to measure motor behavior. To estimate the level of myelination, we performed toluidine blue staining and immunofluorescent staining for OLIG2 and MOG-positive cells. Real-time PCR was used to evaluate the expression of β-catenin, human and mouse Mbp, Mog, and Olig2 genes. Remyelination and motor function improved in mice receiving VPA, hADSCs, and especially VPA/hADSCs compared to the Cup and Sham groups (P < 0.01). Additionally, the number of MOG and OLIG2 positive cells significantly increased in the VPA/hADSCs group compared to the Cup and Sham groups (P < 0.01). The expression of β-catenin, myelin and the other oligodendrocyte-specific genes was significantly higher in the VPA recipient groups. Valproic acid can enhance the differentiation of stem cells into oligodendrocytes, making it a potential candidate for MS treatment.
Keywords
Multiple Sclerosis, valproic acid, human adipose-derived stem cell, neuroprotective agent, remyelination
Introduction
Stem cells (SCs) are undifferentiated cells with the capacity for self-renewal and the potential to differentiate into other cell types (Zakrzewski et al., 2019). Cell therapy using embryo and adult stem cells has recently emerged as an advanced area of scientific research. In this context, adipose-derived stem cells have garnered particular attention due to their abundance in adipose tissue and ease of isolation. These cells can repair damaged tissue through immunomodulatory and neuroprotective effects, achieved by releasing trophic factors, inhibiting inflammation, and creating a microenvironment conducive to the survival of neurons, axons, and oligodendrocytes (Bateman et al., 2018). Previous studies have also demonstrated the beneficial effects of human adipose-derived stem cell (hADSC) transplantation in structural and functional recovery from neurodegenerative disorders such as Multiple Sclerosis (MS) (Ghasemi, 2018; Villoslada, 2016).
MS is a chronic disease characterized by various neurological symptoms resulting from demyelination (Moghadasi, 2020). It is essential to note that, in this abnormal condition, widespread demyelination and the formation of dense glial scars typically occur in various regions of the white matter due to the spread of inflammation. Previously, MS pathology solely focused on the appearance of focal demyelinated plaques in the white matter (Lassmann, 2018). However, it is now recognized that demyelination and glial scar formation can also be observed in the gray matter of the brain and spinal cord (Gajofatto and Benedetti, 2015).
Experimental studies' findings in MS treatment revealed that immunomodulatory drugs and neuroprotective agents can protect neurons and enhance remyelination by reducing central nervous system (CNS) inflammation and cell death. Consequently, the formation and regeneration of myelin have been mimicked as a specific biological process in the CNS through experimental therapies (Villoslada, 2016).
Neurotrophic factors, via the activation of signaling pathways such as phosphoinositide 3-kinase, glycogen synthesis kinase-3β (GSK-3β), mitogen-activated protein kinase, and the nuclear factor-κB pathway, can prevent cell apoptosis, promote cell survival and differentiation, and maintain communication between target cells and neurons. It has been reported that the inactivation of the GSK-3β pathway can lead to brain protection, motor function recovery, neuronal survival, and neurogenesis by stimulating brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), Cyclin D1, and Neuro D1 (Xu et al., 2019; Moon et al., 2018). Valproic acid (VPA), a GSK-3β inhibitor (Zeng et al., 2019), is widely employed clinically as a pharmacological agent in treating neurological diseases. This factor is pivotal in regulating cellular processes, including cell differentiation and proliferation, by influencing intracellular signaling through specific receptors (Moon et al., 2018).
Based on the introduction above, if valproic acid can stimulate the differentiation of stem cells into oligodendrocyte cells, it could be considered a potential candidate for a novel treatment approach for MS. Consequently, this in-vivo study was designed to assess the effects of valproic acid, as a GSK3-β inhibitor, on stem cell differentiation into oligodendrocytes and remyelination in a mouse model of multiple sclerosis induced by cuprizone.
Among the various animal models of MS, cuprizone (Cup) has been used recently to induce MS in animals. Cuprizone, as a toxic compound and a copper chelating agent, can cause demyelination in different areas of the brain such as the corpus callosum, by disrupting the metabolism of oligodendrocytes(Zhen et al., 2017; Kopanitsa et al., 2021). Cup can reduce the expression of myelin-related genes and proteins such as myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein in the carpus callosum (Han et al., 2020). Therefore, in this study, a cuprizone model of MS was used.
Results
Characterization of hADSCs
After cell culture, the hADSCs exhibited an elongated morphology similar to fibroblast cells, confirming their mesenchymal nature. Furthermore, upon examination of the cells under a fluorescent microscope, it was confirmed that approximately 80% were labeled with PKH26.
Effects of hADSCs and VPA on mouse motor function improvement and weight changes
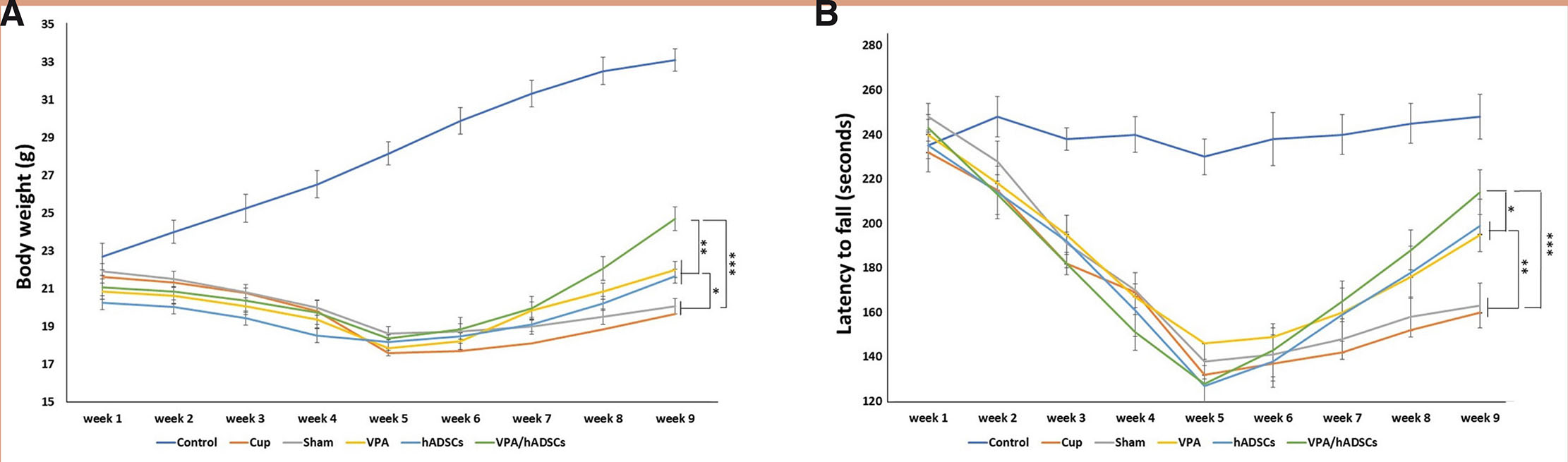
Mouse body weight and motor behavior were assessed and recorded weekly using a hanging wire test. Data evaluation showed that weight loss and a decline in motor function were evident due to the application of cuprizone. However, the use of VPA, hADSCs, and especially VPA/hADSCs (P < 0.05, P < 0.05, P < 0.001, respectively) led to a significant increase in body weight compared to the other groups (Fig. 1A). In general, the groups receiving VPA, hADSCs alone, and in combination demonstrated a significant increase in the hanging time of the mice compared to the untreated groups (P < 0.01, P < 0.01, P < 0.001, respectively). Additionally, there was a statistically significant increase observed in the VPA/hADSCs group when compared to the VPA and hADSCs groups (P < 0.05) (Fig. 1B).
Fig. 1. Body weight changes of mice during the study.
(A) Comparing the body weight of mice showed that the use of cuprizone led to weight loss, and after received of VPA and hADSCs, body weight increased significantly compared with other groups. (B) Hanging wire test also, showed a significant improvement in the motor function of mice after receiving hADSCs and VPA. Groups: Cuprizone (Cup), valproic acid (VPA), human adipose derived stem cells (hADSC), and VPA/hADSC. (*P < 0.05, **P < 0.01, ***P < 0.001).
Histological analysis
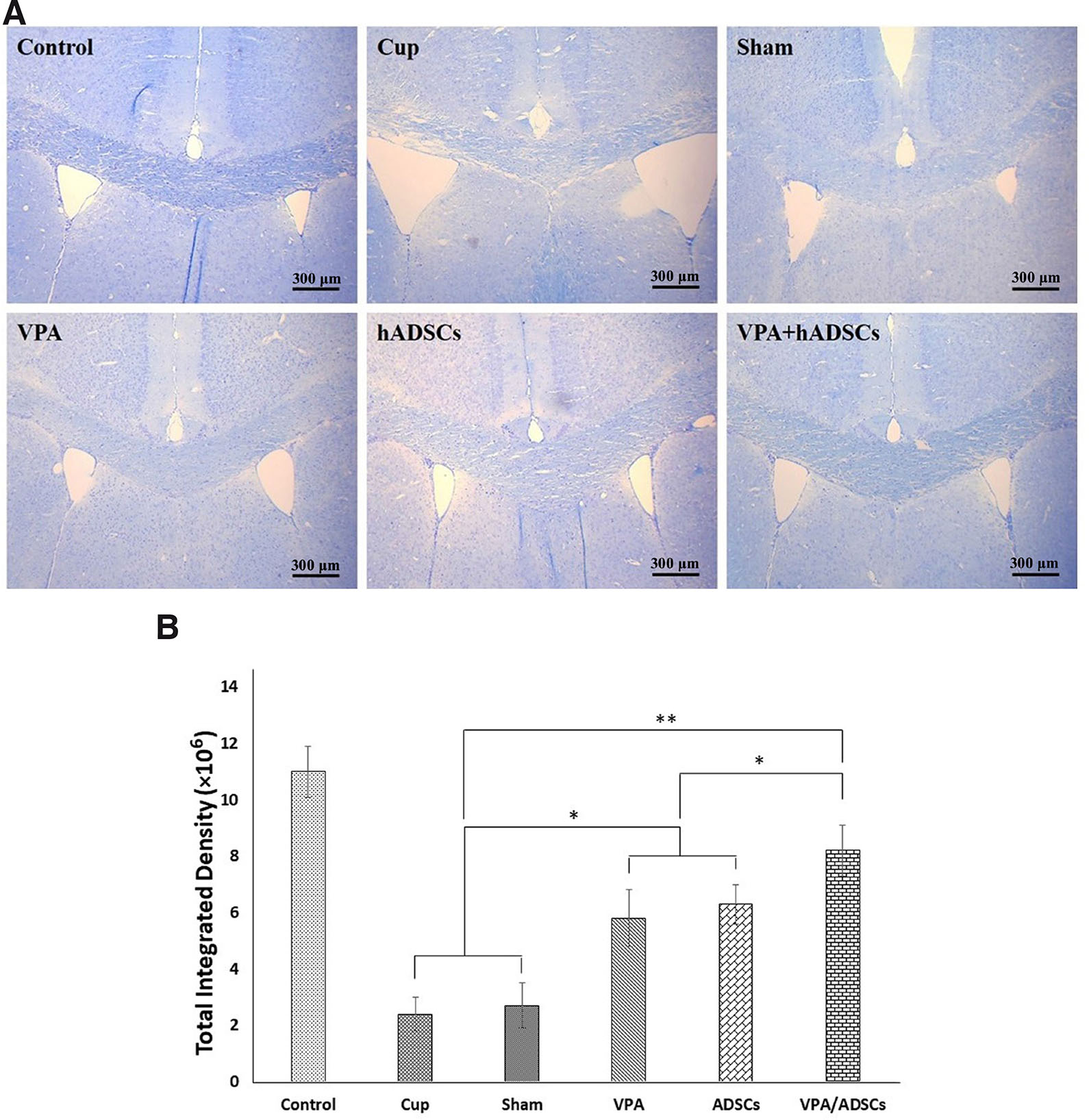
To assess the myelin condition, we stained tissue slides with toluidine blue in the corpus callosum (Fig. 2A). Considerable demyelination was evident in the images of the untreated (Cup and sham) groups. In contrast, myelin density measurement indicated that remyelination occurred using hADSCs or VPA. When comparing the groups, we observed the highest myelin density in the VPA/hADSCs group (*; P<0.01) (Fig. 2B).
Fig. 2. Staining of mouse corpus callosum sections with toluidine blue.
(A) Demyelination was evident in the images of the untreated (Cup and sham) groups. In contrast, myelin density measurement indicated that re-myelination occurred using hADSCs or VPA. (B) Myelin density was assessed using Image J software. After receiving hADSCs and VPA, myelin density increased significantly.
Immunohistochemistry
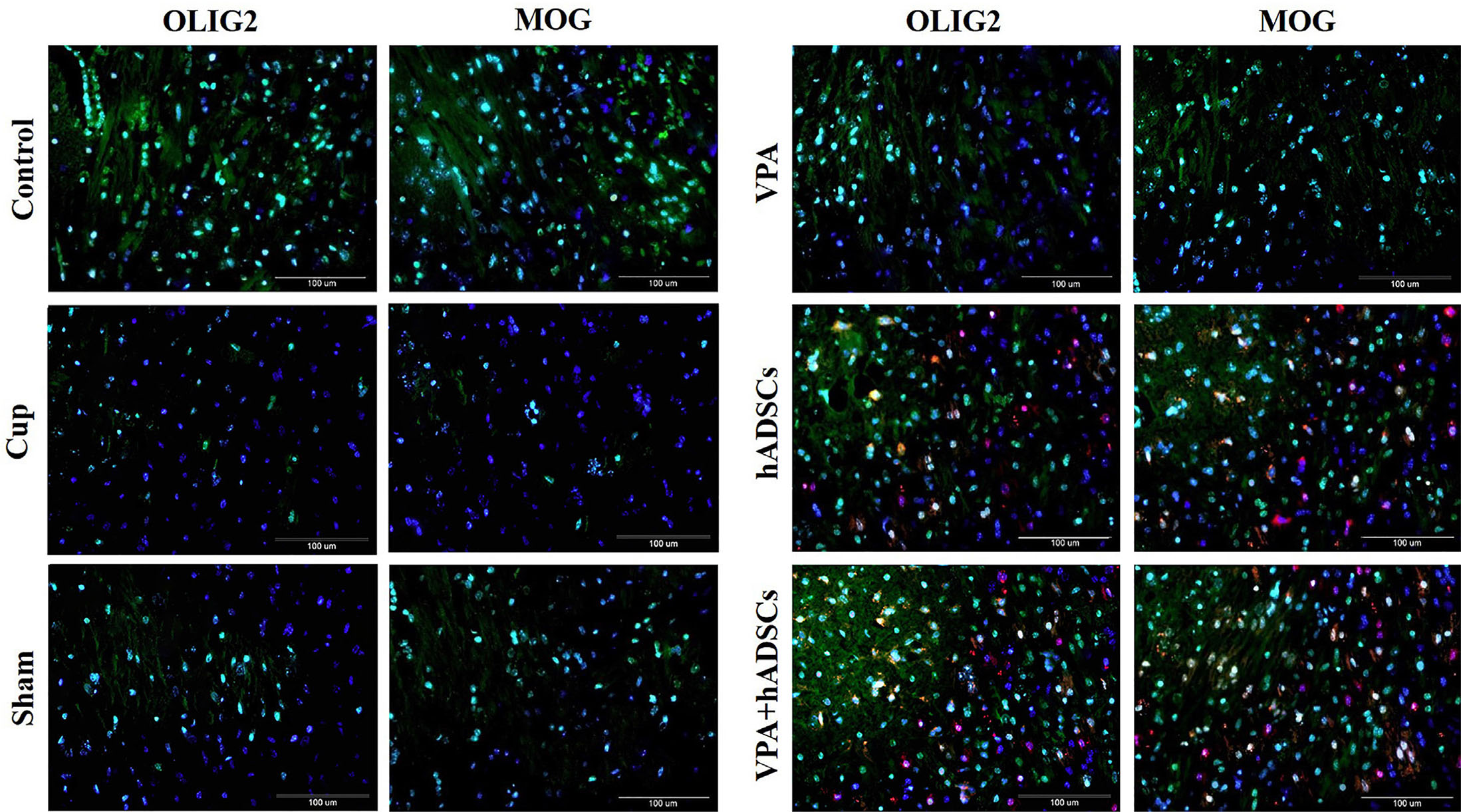
Immunohistochemistry using cell type-specific markers (Fig. 3) revealed an increase in MOG and OLIG2 positive cells in the groups treated with VPA, hADSCs, and VPA/hADSCs compared to the untreated groups (P<0.05). However, no significant difference was observed between the groups receiving VPA and hADSCs compared to the VPA/hADSCs group. The analysis of oligodendrocyte differentiation in the hADSC recipient groups demonstrated that the PKH26/OLIG2+ and PKH26/MOG+ positive cells were significantly fewer in the hADSC group (7.2 ± 0.74% and 8.6 ± 0.65%, respectively) than in the VPA/hADSCs group (13.42 ± 0.91% and 14.8 ± 1.05%, respectively) (P<0.05).
Fig. 3. Immunohistochemical staining of mouse brain sections with oligodendrocyte-specific cell markers.
PKH26- labeled hADSCs (red) were shown around the nucleus, labeled blue with DAPI. The OLIG2 and MOG markers were expressed by PKH26+ cells. Groups: cuprizone (Cup), valproic acid (VPA), human adipose derived stem cells (hADSCs), and VPA/hADSCs. Scale bar, 100 µm.
Increase in the expression of β-catenin, myelin, and oligodendrocyte-specific genes in response to VPA and hADSC treatments
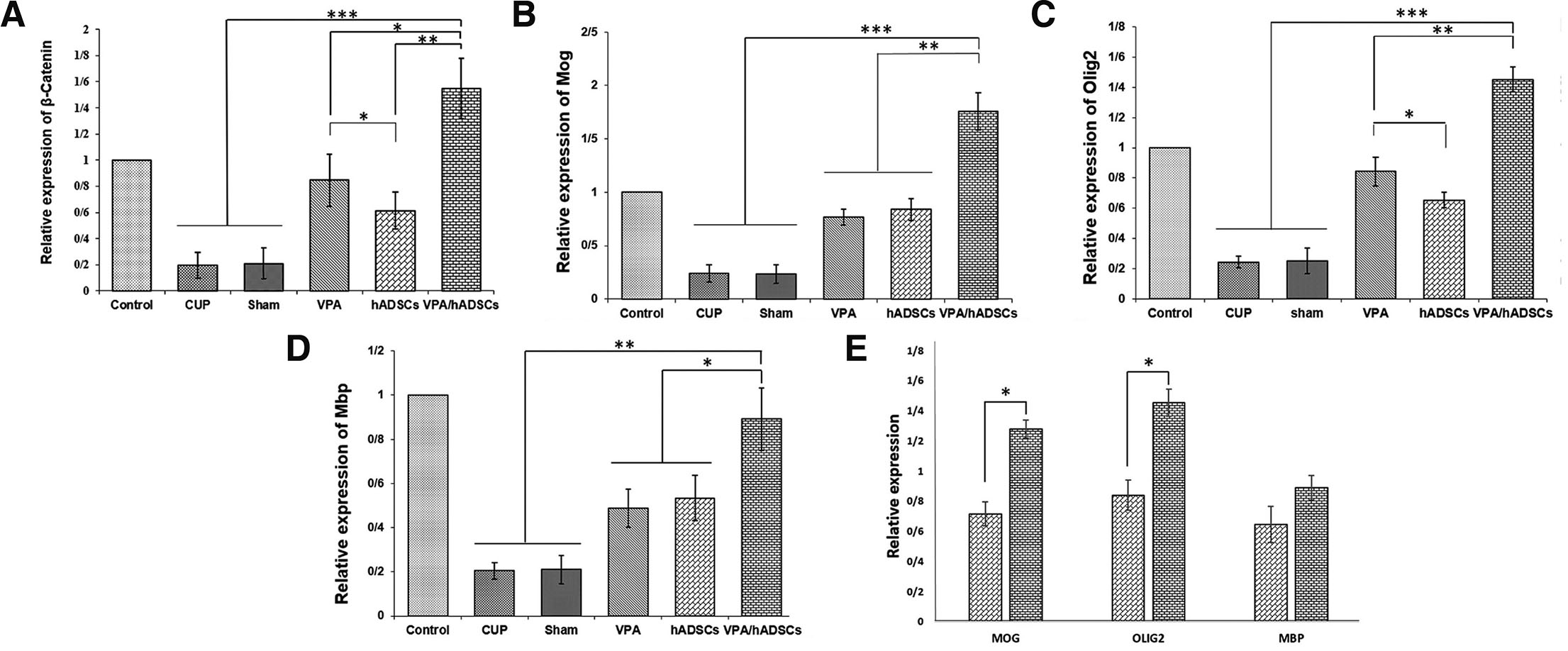
Five weeks after treatment with VPA and hADSCs, the expression levels of β-Catenin, mouse and human Mbp, Mog, and Olig2 were measured using Real-Time PCR. The results showed that the expression of β-catenin, human and mouse myelin, and oligodendrocyte-specific genes was significantly reduced in the cup and sham groups compared to the treated groups. Meanwhile, a significant increase in gene expression was observed in the VPA and hADSCs treated groups, especially in the group receiving VPA and hADSCs simultaneously (Fig. 4). In the end, no significant difference was observed in the expression of the Mbp gene between the hADSCs group and the VPA/hADSCs group (Fig. 4D). However, significant differences for MOG and OLIG2 were found (Fig. 4E).
Fig. 4. The relative expression of β-catenin, myelin and oligodendrocyte-specific genes in the corpus callosum.
(A-D) Results showed a significant upregulation of β-catenin, Mbp, Olig2 and Mog in VPA and hADSC treated groups, compared with untreated groups. (E) Comparison of the expression of human MBP, OLIG2 and MOG found in the hADSC and VPA/hADSC groups. The simultaneous use of VPA/hADSC had a significant increase in the expression of MOG and OLIG2 genes. Groups: Cuprizone (Cup), Valproic Acid (VPA), human Adipose Derived Stem Cells (hADSCs), and VPA/hADSCs. (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
The destruction of the myelin sheath can occur in various CNS disorders, such as multiple sclerosis (MS). In MS disease, the myelin sheath and oligodendrocytes are subject to destruction due to infiltration by immune cells (Dehghan et al., 2016). This abnormal condition can induce severe physical or cognitive disabilities owing to the formation of MS plaques and disturbances in the transmission of nerve impulses. Given that remyelination and recovery in MS are often limited and incomplete, there is a growing concern about the treatment of MS. Current published data suggests that cell-based therapy employing stem cells and neurotrophic factors has introduced a potential new paradigm for MS treatment. For instance, it has been demonstrated that the intravenous injection of ADSCs can promote remyelination in the corpus callosum (Killer et al., 2017).
Moreover, the transplantation of hADSCs into a mouse model of MS has led to improved motor coordination, balance, and spinal cord myelination (Talwadekar et al., 2017). Additionally, applying certain drugs or factors can enhance the differentiation of stem cells into target cells. Recent studies have indicated that combining stem cells with neuroprotective agents such as edaravone, pregnenolone, and VPA can increase the differentiation of oligodendrocytes and induce remyelination (Pazhoohan et al., 2014; Bakhtiari et al., 2021; Ganji et al., 2020).
Other studies report that the motor performance of animals improved with the appropriate dose of PLX3397. It is possible that PLX3397 could maintain myelination by regulating the myelin environment in the MS model. Experimental results have shown that the number of microglia significantly decreased following MSC transplantation. Furthermore, the oligodendrocyte population increased, leading to enhanced remyelination in the corpus callosum of the cuprizone model of MS. Consequently, PLX3397 treatment and MSC injection reduced astrocytosis and microgliosis. Another study has confirmed that applying PLX for 21 days increased remyelination by enhancing oligodendrocytes and reducing microglia in the demyelination model (Tahmasebi et al., 2021b; Tahmasebi et al., 2019; Tahmasebi et al., 2021a).
Previous studies have demonstrated that VPA plays a significant role in the regeneration, remyelination, and regulation of the peripheral and central nervous systems through intracellular signaling via specific receptors (Pazhoohan et al., 2014; Jang and Jeong, 2018; Zhu et al., 2021; Azuchi et al., 2017). Since neurotrophic factors are emerging as therapeutic agents for restoring and maintaining the function of oligodendrocytes, our research evaluated the differentiation of stem cells into oligodendrocytes and remyelination of the corpus callosum area in cuprizone-induced mice 5 weeks after treatment with hADSCs and VPA.
We have demonstrated that VPA can mitigate the adverse effects of cuprizone, including demyelination, oligodendrocyte death, muscle weakness, and weight loss in a mouse model of MS. Cuprizone can induce apoptosis of oligodendrocytes by inhibiting cytochrome oxidase and inducing mitochondrial disorder in the energy production cycle (Mohammadi-Rad et al., 2019). However, VPA can mitigate these adverse effects through its neuroprotective properties and by enhancing remyelination via the secretion of neurotrophic factors and neuroprotective functions (Chen et al., 2016; Ghasemi et al., 2014).
Furthermore, injecting hADSCs and applying VPA can increase the mean percentage of Olig2 and MOG-positive cells. According to these findings, VPA may operate within the therapeutic range by either promoting the differentiation of hADSCs into oligodendrocytes or enhancing cell proliferation and protection. Consequently, it increases the population of myelin-generating oligodendrocytes, thereby improving motor behavior and remyelination. Other results have indicated that VPA can play a significant role in nerve regeneration by promoting the differentiation of ADSCs into neurons. Additionally, in the mouse MS model, the expression of Olig2 and MBP genes increased with hADSCs, leading to stimulation of remyelination and improved motor behavior (Bakhtiari et al., 2021; Kim et al., 2015; Santos et al., 2020).
It is worth noting that VPA, as a GSK3-β inhibitor, can regulate the fate of stem cells by altering the expression of specific genes in the Wnt/GSK3-β molecular pathway, such as the transcription of the β-catenin protein (Kim et al., 2015; Soleimani and Ghasemi, 2017). Moreover, increasing the level of β-catenin in the cell appears to be a potential new therapeutic target for treating MS (Chiu and Chuang, 2011). β-catenin is crucial in the Wnt pathway for initiating cellular processes such as proliferation, differentiation, and maturation of stem cells. Additionally, it plays a neuroprotective role (Kühl and Kühl, 2013; Miki et al., 2011). As depicted in Fig. 4E, in VPA-treated groups, the expression of the β-catenin gene and the genes associated with oligodendrocytes (Mog and Olig2) and myelin production (Mbp) increased.
Therefore, VPA may inhibit the phosphorylation of β-catenin through the inactivation of GSK-3β, which in turn could enhance the expression of anti-apoptotic genes and the transcription of growth factors by raising the cytoplasmic level of β-catenin and facilitating its translocation into the nucleus. As a result, VPA improves motor behavior and enhances remyelination by reducing oligodendrocyte death, promoting cell survival and proliferation, and facilitating the differentiation of transplanted cells into myelin-producing cells (Fig. 5).
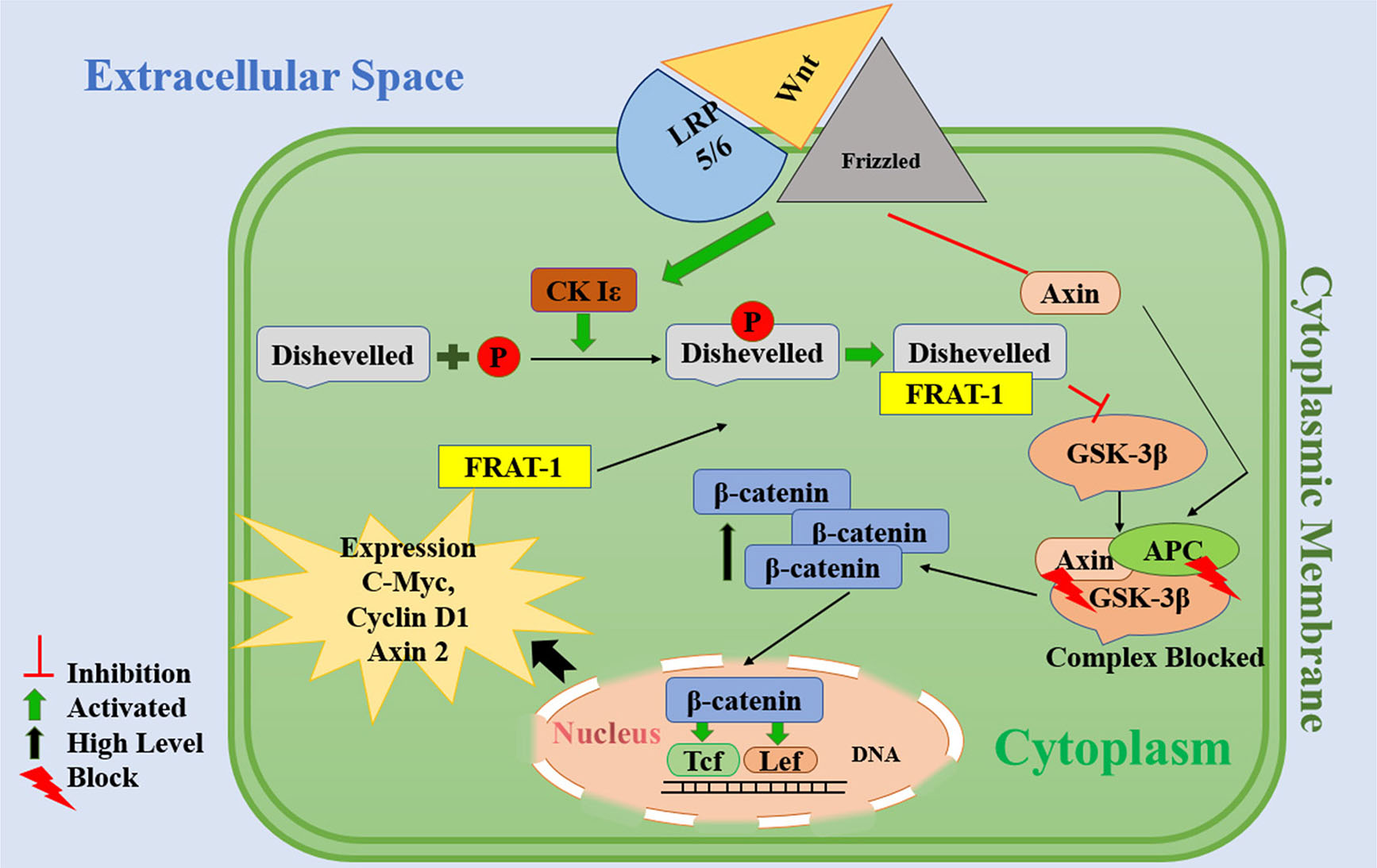
Fig. 5. Proposed mechanism of action of valproic acid.
The Wnt signaling pathway is activated upon wnt ligand binding to the transmembrane receptor Frizzled and co-receptor LRP-5/6. After forming theWnt-Frizzled-LRP5/6 complex, Dishevelled (DVL) is employed/recruited. Subsequently, DVL is phosphorylated by casein kinase Iε (Ck Iε) and connection of DVL with Frat inhibits GSK-3β activity. Additionally, the LRP5/6 receptor from the Wnt-Frizzled-LRP5/6 complex will degrade Axin. The complex of adenomatous polyposis coli (APC), GSK-3β and Axin is not formed and as a result, GSK-3β is inhibited. Thereafter, the inactivation of this complex causes the blockage of β-catenin protein phosphorylation and promotes the cytoplasmic concentration of β-catenin. When β-catenin passes through the nucleus membrane, it activates the Tcf / Lef transcription factor leading to the transcription and expression of cyclin D1, Axin 2 and c-Myc.
In summary, our research results demonstrate that valproic acid can effectively promote the differentiation of human adipose-derived stem cells into oligodendrocytes and enhance remyelination in multiple sclerosis (MS) diseases by inactivating GSK-3β function and phosphorylating β-catenin. However, further studies are required to investigate the detailed mechanism underlying these therapeutic effects.
Materials and Methods
Experimental animals
We employed adult female C57BL/6 mice (Laboratory Animal Center, Royan Center, Isfahan, Iran), 8 weeks old and weighing 18 - 22 g, for our study. This research received approval from the IUMS Ethical Committee and was conducted using cuprizone mice models at the Central Laboratory of Isfahan University of Medical Sciences (Liu et al., 2018). The mice were provided free access to food and water and maintained under suitable health conditions and temperature control (23°C ± 2°C). They were divided into five groups: cuprizone (Cup, n=8), sham (n=8, received normal saline for 4 weeks intraperitoneally (IP)), human adipose-derived stem cells (n=8, 1× 106 labeled hADSCs, administered intravenously (IV)), valproic acid (Azuchi et al., 2017) (VPA, n=8, 300 mg/kg/day VPA, IP), and VPA/hADSCs (n=8, 300 mg/kg/day VPA, IP for 4 weeks and 1× 106 labeled hADSCs, IV) groups. A healthy control group (n=8) was also included for comparison purposes.
Induction of demyelination
All mice (except the healthy control group) were administered a dose of cuprizone. They received 0.2% cuprizone (Sigma-Aldrich Inc. C9012) for 5 weeks to induce demyelination (Liu et al., 2018). Throughout the study, we measured the body weight and monitored behavioral changes in all mice to assess the effects of cuprizone.
Thawing and culture of hADSCs
We retrieved the frozen cells (hADSCs in passages 2: P2) from the nitrogen tank and thawed them following the procedure outlined in our previous study (Bakhtiari et al., 2021). Subsequently, we placed the cell suspension into T75 flasks and cultured the cells in the presence of DMEM/F12 (Bioidia, BI1027) containing 10% fetal bovine serum (FBS) (Gibco, 10270106) and a penicillin/streptomycin solution. We used 1× 106 labeled hADSCs from passages 4 (P4) for injection into the tail vein of the mice.
Cell membrane labeling with PKH26
Seventy-two hours before cell transplantation, human Adipose-Derived Stem Cells (hADSCs) were labeled with the in vitro cell membrane labeling agent PKH-26 (Sigma-Aldrich, MINI26). To achieve this, we placed cells in a conical tube and washed them once with a serum-free medium to remove proteins and lipids. Subsequently, we centrifuged the cells at 1500 rpm for 5 minutes. PKH26 was added to the cells washed with a phosphate buffer solution (PBS). After 5 minutes, 1% bovine serum albumin (BSA) (Sigma-Aldrich A2153) was utilized to halt the cell labeling process. The cells were washed three times with DMEM/F12 medium to eliminate any excess dye, and fluorescent microscopy (Olympus BX51, Japan) was employed to assess staining efficiency. (Ganji et al., 2020; Ghasemi et al., 2014; Razavi et al., 2018)
Behavioral test (Wire-Hanging test)
The wire-hanging test was conducted throughout the study to assess motor behavior and muscular strength. For this purpose, a metal wire with a 2 mm diameter was utilized. Mice were suspended from the wire for 5 minutes. If a mouse lost its balance and fell off the wire during the experiment, it was placed on the wire twice, and the hanging time was calculated. Mice displaying a decrease in hanging time of more than 60 seconds were categorized as having significant motor dysfunction compared to before the injury, while those with slight motor dysfunction exhibited a decrease in the longest hanging time of 30 to 60 seconds. Mice with a decrease in the longest hanging time of less than 30 seconds were considered to have no motor disability (Yamazaki et al., 2021).
Toluidine blue staining
Immediately following the study's conclusion, mice were deeply anesthetized using ketamine/xylazine, and cardiac perfusion was performed to fix the brain, as previously described (Yu et al., 2017). The brains of the mice were then immersed in 4% paraformaldehyde for 24 hours and subsequently embedded in paraffin. Sections with a thickness of 5 μm were prepared using a microtome. Finally, the slides were stained with toluidine blue, and an Olympus Provis light microscope was employed to examine the stained samples (Zhang et al., 2020).
Immunohistochemistry (IHC)
In this study, Olig2 and myelin oligodendrocyte glycoprotein (MOG) were utilized as specific markers for oligodendrocytes. Tissue samples were deparaffinized and dehydrated. In order to evaluate oligodendrocyte and myelin markers, we prepared 5 µm thick sections of the corpus callosum using a conventional microtome. Antigen retrieval was done by adding sodium citrate buffer and 1 mM EDTA buffer for 10 minutes at 90°C (Gibco, USA). Primary antibodies, including anti-Olig2 antibody (1:1000; Abcam, Cambridge, MA, USA) and anti-MOG antibody (1:1000; Abcam, Cambridge, MA, USA), were applied, and the slides were incubated overnight at 4°C. Following a PBS wash, the slides were incubated at room temperature for 1 hour with FITC-conjugated secondary antibodies (1:500; Abcam, Cambridge, MA, USA). Subsequently, sections were stained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, D9542) for 2 minutes to label cell nuclei. Finally, using a fluorescent microscope and ImageJ software, the average percentage of cells expressing MOG and Olig2 markers was determined from 200 cells per slide (Bakhtiari et al., 2021).
RT-PCR analysis
The levels of β-catenin, human and mouse Mbp, Mog, and Olig2 genes in CC were assessed using real-time RT-PCR. After RNA extraction with the Total RNA Prep Kit (BIOFACT, RP101–050, Korea), 2 µg of RNA were utilized for cDNA synthesis using the BioFact™ 5X RT Pre-Mix cDNA Synthesis Kit (BIOFACT, 25081), following the manufacturer's protocol. The expression of the target genes was measured using the BioFact™ 2X Real-Time PCR Master Mix Kit (BIOFACT, DQ385–40) in a total volume of 20 μL, containing Power SYBR Green master mix (2x), forward and reverse primers (0.5 μM), cDNA (30 ng/μL), and H2O, with the following cycling conditions on the Step One Plus™ quantitative real-time PCR detection system (Applied Biosystems): 1 cycle of denaturation (95˚C for 5 minutes), followed by a 40-cycle amplification (at 95˚C for 15 seconds) and the extension cycle (at 60˚C for 40 seconds). β-actin was used as the housekeeping gene. The sequences of the primers used are listed in Table 1. (Bakhtiari et al., 2021; Razavi et al., 2021)
Table 1
The list of primers used in this study
| Gene | Primer sequences |
|---|---|
| В-actin-m-F | 5′-CGGTTCCGATGCCCTGAGGCTCTT-3′ |
| В-actin-m-R | 5′-CGTCACACTTCATGATGGAATTGA-3′ |
| В-ACTIN-H-F | 5′-AGCCTCGCCTTTGCCGATCC-3′ |
| В-ACTIN-H-R | 5′-ACATGCCGGAGCCGTTGTCG-3′ |
| Mbp-m-F | 5′-AACATTGTGACACCTCGAACA-3′ |
| Mbp-m-R | 5′-TGTCTCTTCC TCCCCAGCT-3′ |
| MBP-H-F | 5′-ACTATCTCTTCCTCCCAGCTTAAAAA-3′ |
| MBP-H-R | 5′-TCCGACTATAAATCGGCTCACA-3′ |
| Mog-m-F | 5′-TGATTTCCCTCCCTCAACTG-3′ |
| Mog-m-R | 5′-CGTATCCTGGTTGGCAGAAT-3′ |
| MOG-H-F | 5′-ACCAGGCACCTGAATATCGG-3′ |
| MOG -H-R | 5′-CAGGGCTCACCCAGTAGAAAG-3′ |
| Olig2-m-F | 5′-TGGAGAGATGCGTTCGTTCC-3′ |
| Olig2-m-R | 5′-GTGCTCTGCGTCTCGTCTAA -3′ |
| OLIG2-H-F | 5′-AGATCGACGCGACACCAGCG-3′ |
| OLIG2-H-R | 5′-TCGGACCCGAAAATCTGGATGCG-3′ |
| βcatenin-m-F | 5′-GTACGCACCATGCAGAATAC-3′ |
Statistical analysis
Statistical differences between the groups were determined using a one-way ANOVA followed by the LSD test through SPSS software version 25.0. Results were presented as mean ± standard error, and p-values less than 0.05 were considered significant.
Acknowledgements
Our research was performed as a section of the Ph.D. thesis and we acknowledge for providing the facility and funding from the Isfahan University of Medical Sciences.
Abbreviations
CC, Corpus callosum ; CNS, Central nervous system ; CUP, Cuprizone ; GSK3-β, Glycogen synthase kinase-3 beta ; hADSCs, Human Adipose-Derived Stem Cells ; iP, Intraperitoneally ; MBP, Myelin basic protein ; MOG, Myelin oligodendrocyte glycoprotein ; MS, Multiple Sclerosis ; SCs, Stem cells ; VPA, Valproic acid ;Declarations
Funding
Isfahan University of Medical Sciences supported this work (grant number: 399831).
Author contributions
The conception, design, statistical analysis, and drafting of the manuscript were participated by Sahar Ghosouri and Nazem Ghasemi. Sahar Ghosouri and Mohammad Bakhtiari collected data. Mitra Soleimani participated in revision of the manuscript.
Ethics approval
Female C57BL/6 mice model were used in this experimental of study on of MS at the Central Laboratory of Isfahan University of Medical Sciences. however, the Ethics Committee approved all process of research were carried out according to the guidelines of the Iranian Committee of Animal care (Ethics#IR.MUI.MED.REC.1399.925).
Availability of Data and Materials
Data are available from the corresponding authors upon reasonable request.
References
Azuchi Y., Kimura A., Guo X., Akiyama G., Noro T., Harada C., Nishigaki A., Namekata K., Harada T. (2017). Valproic acid and ASK1 deficiency ameliorate optic neuritis and neurodegeneration in an animal model of multiple sclerosis. Neuroscience Letters 639: 82-87.
Bakhtiari M., Ghasemi N., Salehi H., Amirpour N., Kazemi M., Mardani M. (2021). Evaluation of Edaravone effects on the differentiation of human adipose derived stem cells into oligodendrocyte cells in multiple sclerosis disease in rats. Life Sciences 282: 119812.
Bateman M. E., Strong A. L., Gimble J. M., Bunnell B. A. (2018). Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells 36: 1311-1328.
Chen Y., Weng J., Han D., Chen B., Ma M., Yu Y., Li M., Liu Z., Zhang P., Jiang B., (2016). GSK3β inhibition accelerates axon debris clearance and new axon remyelination. American journal of translational research 8: 5410-5420.
Chiu C. T., Chuang D. M., (2011). Neuroprotective action of lithium in disorders of the central nervous system. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences 36: 461-476.
Dehghan S., Hesaraki M., Soleimani M., Mirnajafi-Zadeh J., Fathollahi Y., Javan M. (2016). Oct4 transcription factor in conjunction with valproic acid accelerates myelin repair in demyelinated optic chiasm in mice. Neuroscience 318: 178-189.
Gajofatto A., Benedetti M. D. (2015). Treatment strategies for multiple sclerosis: When to start, when to change, when to stop?. World Journal of Clinical Cases 3: 545.
Ganji R., Razavi S., Ghasemi N., Mardani M. (2020). Improvement of Remyelination in Demyelinated Corpus Callosum Using Human Adipose-Derived Stem Cells (hADSCs) and Pregnenolone in the Cuprizone Rat Model of Multiple Sclerosis. Journal of Molecular Neuroscience 70: 1088-1099.
Ghasemi N., (2018). The Evaluation of Astaxanthin Effects on Differentiation of Human Adipose Derived Stem Cells into Oligodendrocyte Precursor Cells. Avicenna journal of medical biotechnology 10: 69-74.
Ghasemi N., Razavi S., Mardani M., Esfandiari E., Salehi H., Zarkesh Esfahani S. H., (2014). Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Molecular biotechnology 56: 470-478.
Han S. R., Kang Y. H., Yoo S.M., Lee M.S., Lee S.H. (2020). Diverse Gene Expressions in the Prediction of Cuprizone-Induced Demyelination. Neurotoxicity Research 37: 732-742.
Jang S., Jeong H.S. (2018). Histone deacetylase inhibition-mediated neuronal differentiation via the Wnt signaling pathway in human adipose tissue-derived mesenchymal stem cells. Neuroscience Letters 668: 24-30.
Killer M. C., Nold P., Henkenius K., Fritz L., Riedlinger T., Barckhausen C., Frech M., Hackstein H., Neubauer A., Brendel C. (2017). Immunosuppressive capacity of mesenchymal stem cells correlates with metabolic activity and can be enhanced by valproic acid. Stem Cell Research & Therapy 8: 100.
Kim H., Kim S., Song Y., Kim W., Ying Q.L., Jho E. (2015). Dual Function of Wnt Signaling during Neuronal Differentiation of Mouse Embryonic Stem Cells. Stem Cells International 2015: 1-10.
Kopanitsa M. V., Lehtimäki K. K., Forsman M., Suhonen A., Koponen J., Piiponniemi T. O., Kärkkäinen A.M., Pavlidi P., Shatillo A., Sweeney P. J., Merenlender‐Wagner A., Kaye J., Orbach A., Nurmi A. (2021). Cognitive disturbances in the cuprizone model of multiple sclerosis. Genes, Brain and Behavior 20: e12663.
Kühl S. J., Kühl M. (2013). On the role of Wnt/β-catenin signaling in stem cells. Biochimica et Biophysica Acta (BBA) - General Subjects 1830: 2297-2306.
Lassmann H. (2018). Multiple Sclerosis Pathology. Cold Spring Harbor Perspectives in Medicine 8: a028936.
Liu M., Liu X., Wang L., Wang Y., Dong F., Wu J., Qu X., Liu Y., Liu Z., Fan H., Yao R. (2018). TRPV4 Inhibition Improved Myelination and Reduced Glia Reactivity and Inflammation in a Cuprizone-Induced Mouse Model of Demyelination. Frontiers in Cellular Neuroscience 12: 392.
Miki T., Yasuda S., Kahn M. (2011). Wnt/β-catenin Signaling in Embryonic Stem Cell Self-renewal and Somatic Cell Reprogramming. Stem Cell Reviews and Reports 7: 836-846.
Moghadasi A. N., (2020). History of Multiple Sclerosis in Iran. Archives of Iranian medicine 23: 211-215.
Mohammadi-Rad M., Ghasemi N., Aliomrani M. (2019). Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Research in Pharmaceutical Sciences 14: 424.
Moon B.S., Lu W., Park H. J. (2018). Valproic acid promotes the neuronal differentiation of spiral ganglion neural stem cells with robust axonal growth. Biochemical and Biophysical Research Communications 503: 2728-2735.
Pazhoohan S., Satarian L., Asghari A.A., Salimi M., Kiani S., Mani A.R., Javan M. (2014). Valproic Acid Attenuates Disease Symptoms and Increases Endogenous Myelin Repair by Recruiting Neural Stem Cells and Oligodendrocyte Progenitors in Experimental Autoimmune Encephalomyelitis. Neurodegenerative Diseases 13: 45-52.
Razavi S., Ghasemi N., Mardani M., Salehi H., (2018). Co-Transplantation of Human Neurotrophic Factor Secreting Cells and Adipose-Derived Stem Cells in Rat Model of Multiple Sclerosis. Cell journal 20: 46-52.
Razavi S., Jahromi M., Vatankhah E., Seyedebrahimi R. (2021). Differential effects of rat ADSCs encapsulation in fibrin matrix and combination delivery of BDNF and Gold nanoparticles on peripheral nerve regeneration. BMC Neuroscience 22: 1-16.
Santos J., Hubert T., Milthorpe B. K. (2020). Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways. Cells 9: 619.
Soleimani M., Ghasemi N., (2017). Lithium Chloride can Induce Differentiation of Human Immortalized RenVm Cells into Dopaminergic Neurons. Avicenna journal of medical biotechnology 9: 176-180.
Tahmasebi F., Barati S., Kashani I. R. (2021a). Effect of CSF1R inhibitor on glial cells population and remyelination in the cuprizone model. Neuropeptides 89: 102179.
Tahmasebi F., Pasbakhsh P., Barati S., Madadi S., Kashani I. R. (2021b). The effect of microglial ablation and mesenchymal stem cell transplantation on a cuprizone‐induced demyelination model. Journal of Cellular Physiology 236: 3552-3564.
Tahmasebi F., Pasbakhsh P., Mortezaee K., Madadi S., Barati S., Kashani I. R. (2019). Effect of the CSF1R inhibitor PLX3397 on remyelination of corpus callosum in a cuprizone‐induced demyelination mouse model. Journal of Cellular Biochemistry 120: 10576-10586.
Talwadekar M., Fernandes S., Kale V., Limaye L. (2017). Valproic acid enhances the neural differentiation of human placenta derived-mesenchymal stem cells in vitro . Journal of Tissue Engineering and Regenerative Medicine 11: 3111-3123.
Villoslada P. (2016). Neuroprotective therapies for multiple sclerosis and other demyelinating diseases. Multiple Sclerosis and Demyelinating Disorders 1: 1-11.
Xu D., Hou K., Li F., Chen S., Fang W., Li Y. (2019). XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/β-catenin signaling dependent way. Life Sciences 235: 116844.
Yamazaki R., Ohno N., Huang J. K. (2021). Acute motor deficit and subsequent remyelination‐associated recovery following internal capsule demyelination in mice. Journal of Neurochemistry 156: 917-928.
Yu Q., Hui R., Park J., Huang Y., Kusnecov A. W., Dreyfus C. F., Zhou R. (2017). Strain differences in cuprizone induced demyelination. Cell & Bioscience 7: 59.
Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. (2019). Stem cells: past, present, and future. Stem Cell Research & Therapy 10: 68.
Zeng Q., Long Z., Feng M., Zhao Y., Luo S., Wang K., Wang Y., Yang G., He G. (2019). Valproic Acid Stimulates Hippocampal Neurogenesis via Activating the Wnt/β-Catenin Signaling Pathway in the APP/PS1/Nestin-GFP Triple Transgenic Mouse Model of Alzheimer’s Disease. Frontiers in Aging Neuroscience 11: 62.
Zhang N., Liu C., Zhang R., Jin L., Yin X., Zheng X., Siebert H.C., Li Y., Wang Z., Loers G., Petridis A. K. (2020). Amelioration of clinical course and demyelination in the cuprizone mouse model in relation to ketogenic diet. Food & Function 11: 5647-5663.
Zhen W., Liu A., Lu J., Zhang W., Tattersall D., Wang J. (2017). An Alternative Cuprizone-Induced Demyelination and Remyelination Mouse Model. ASN Neuro 9: 175909141772517.
Zhu X., Yao Y., Hu Y., Yang J., Zhang C., He Y., Zhang A., Liu X., Zhang C., Gan G. (2021). Valproic acid suppresses cuprizone-induced hippocampal demyelination and anxiety-like behavior by promoting cholesterol biosynthesis. Neurobiology of Disease 158: 105489.