Int. J. Dev. Biol. 68: 1 - 7 (2024)
Estrogen signaling in development: recent insights from the zebrafish
Open Access | Review | Published: 22 February 2024
Abstract
While traditionally recognized as a sex hormone, estrogen has a potent effect on the development of tissues beyond those of the reproductive system. Estrogen synthesis enzymes and estrogen receptors are broadly expressed in vertebrate tissues, further indicating their importance in various processes. These include the tissues of the zebrafish, which is a particularly suitable model for studying early development due to its rapid ex utero ontogeny and conserved genetic and cellular composition with other vertebrates. In this review, we provide readers with an overview of estrogen signaling, discuss important attributes of the zebrafish animal model with a special focus on the kidney, and explore recent insights from zebrafish studies about the roles of estrogen signaling in organogenesis across germ layer derivatives that range from the kidney to the brain and liver.
Keywords
estrogen, estrogen receptor, development, kidney, xenoestrogen, zebrafish
Introduction
Estrogen signaling has long been appreciated in the context of gonadal development and function, and, in more recent years, has been identified as a regulator of other tissues. In this review, we first provide readers with a broad overview of estrogen biosynthesis and signaling. Elucidating the role of estrogen in development is especially challenging, as cell culture models preclude the evaluation of multiple tissues simultaneously and mammalian models develop in utero, thereby preventing high throughput analysis. Here, we secondly discuss how the zebrafish, Danio rerio, offers an alternative to circumvent these barriers and is extremely relevant because it shares high genetic conservation with humans. Thirdly, in this review we highlight recent studies that have uncovered novel roles of estrogen signaling in the zebrafish, with a special focus on the kidney. Finally, we review how the zebrafish has been employed to gain fascinating new insights into the developmental mechanisms that control formation of several other organs, including the blood, brain and liver.
Overview of steroidogenesis
17β-estradiol (E2) is the dominant form of estrogen in vertebrates, but two other estrogenic compounds are also naturally occurring: estrone (E1) and estriol (E3) (Kuiper et al., 1997; Baker, 2013). All three of these compounds are derived from cholesterol. The first step in steroidogenesis involves converting cholesterol to androstenedione via CYP11A1 CYP17A1, and 3β-HSD (HSD3B1). Next, aromatase (CYP19A1) synthesizes E1 from androstenedione. 17β-hydroxysteroid dehydrogenase 1 (HSD1) can then convert E1 into E2. Alternatively, aromatase can use testosterone to form E2. E1 may also be further metabolized to form 16α-hydroxyestrone, which is converted to E3 by 17β-HSD1 (Luu-The, 2013).
Like many processes in development, synthesis of estrogen is spatiotemporally controlled. During human development, E3 is produced in the placenta during pregnancy by conversion of dehydroepiandrosterone (DHEA) rather than 16α-hydroxyestrone (Bondesson et al., 2015). In premenopausal women, estrogen is primarily made in the ovaries, but in male adults and postmenopausal women, estrogen can also be produced in adipose tissue, bone osteoblasts and chondrocytes, smooth muscle, and in the brain (Simpson, 2003). It is worth noting that humans use tissue specific promoters to drive expression of aromatase—the primary enzyme responsible for estrogen synthesis—in these peripheral tissues, but mice lack the majority of these promoters. Mice, however, do retain the ability to produce estrogen in the brain (Stanić et al., 2014). In zebrafish, aromatase expression begins increases at approximately 3-4 days post fertilization (Trant et al., 2001). This is likely due to the presence of E2 at high concentrations in the yolk ball, which suggests that endogenous estrogen production is not needed until a few days after fertilization (Carroll et al., 2014). Aromatases are also differentially expressed in adult zebrafish, depending on sex and tissue (Sawyer et al., 2006). The broad expression of aromatase enzymes suggests that estrogen signaling does indeed play a role outside of gonad formation, and additional studies are needed to interrogate how these signals are regulated.
Estrogen receptors
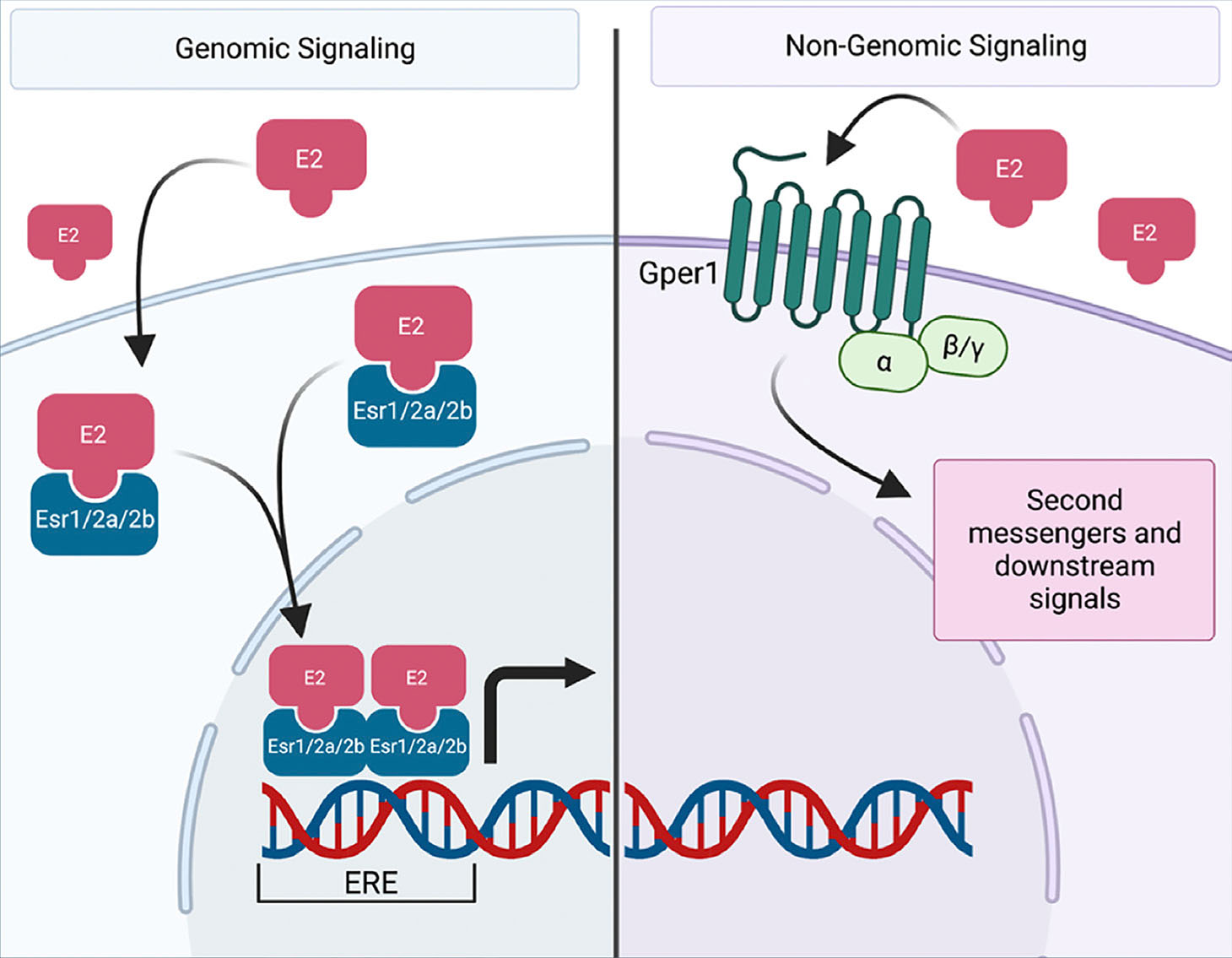
Once estrogen is produced, it can bind three unique receptors in mammalian cells: ESR1 (Esr1 in zebrafish), ESR2 (Esr2a and Esr2b in zebrafish), and GPER (Gper1 in zebrafish) (Bondesson et al., 2015; Thomas et al., 2010; Amenyogbe et al., 2020; Bertrand et al., 2007). ESR1 and ESR2 are ligand activated transcription factors whereas GPER acts as a G protein-coupled receptor that exists on the surface of the cell that can bind estrogen and lead to downstream non-genomic effects (Fig. 1). The nuclear receptors share a similar structure, comprised of a DNA binding domain (DBD), followed by a hinge region which connects the ligand binding domain, flanked by an N-terminal trans-activation domain (Heldring et al., 2007). Estrogen, as a steroid, can diffuse into the cell without the facilitation of a transmembrane protein. Upon entering the cell, it can bind ESR1 or ESR2, both of which remain in the cytoplasm in the absence of a ligand. Upon binding, these receptors dimerize and translocate to the nucleus. There, they can interact with various coactivators, bind to the estrogen response element (ERE), and affect transcription (Farooq, 2015).
Fig. 1. Canonical estrogen signaling in zebrafish.
During genomic signaling (left), estrogen (E2) or other xenoestrogens can directly enter the cell and bind to estrogen receptors. In the zebrafish, these includes receptors Esr1, Esr2a, or Esr2b. Acting as ligand-activated transcription factors, the receptors dimerize and translocate to the nucleus where they bind to the estrogen response element (ERE) and affect transcription. During non-genomic signaling (right), estrogen or other xenoestrogens can bind to a g-protein receptor, Gper1 in the zebrafish. This activates second messengers and various downstream signals. Created with BioRender.com.
The structure of these receptors is highly conserved among vertebrates. Clones of the receptors derived from mouse uterine samples found that homology with rat, human, and chicken estrogen receptors had homology of 97%, 88%, and 77%, respectively (White et al., 1987). Using the most updated zebrafish assembly (Grcz11), homology between human and zebrafish estrogen receptors hovers around 50%. However, the highly conserved functional domains (DBD and LBD) have sequence homology upwards of 70% (Bondesson et al., 2015).
Similar to aromatase, estrogen receptors have been detected in a variety of tissues. In the context of development, estrogen receptors were detected in a human fetus in several developing organs (Brandenberger et al., 1997). Intriguingly, Esr1 and Esr2 were not expressed at identical levels across tissues, further suggesting that these receptors play distinct roles. For example, Esr2 was highly expressed at week 19 in the spleen, while only low levels of Esr1 were detected. Other tissues that express Esr1 and Esr2 include the kidney, stomach, heart, and gonads (Brandenberger et al., 1997). In the context of murine embryogenesis, Esr1, Esr2, and aromatase are expressed in the oocyte. Upon fertilization, aromatase expression diminishes until post-gastrulation. Esr1 and Esr2 expression is present in the early zygote and is then reduced until Esr1 expression spikes during gastrulation. Both receptors see an increase in expression postgastrulation (Bondesson et al., 2015). Zebrafish have a single orthologue of Esr1, esr1, and two orthologues of Esr2, esr2a and esr2b, due to a genome duplication event. All three of these receptors are expressed in the brain, pituitary, liver, and gonads of adult animals (Menuet et al., 2002; Gorelick and Halpern, 2011; Chaturantabut et al., 2020). esr2a is also expressed in neuromast cells, and esr2a and esr2b are expressed more in the intestine (Froehlicher et al., 2009; Menuet et al., 2002). Such precise control of expression further points towards unique regulatory roles for these receptors and aromatase.
Mammalian GPER also shares high sequence homology with zebrafish, similar to the other estrogen receptors (Liu et al., 2009). However, the structure and signaling pathway of this receptor differs from that of ESR1 and ESR2. Like other G protein-coupled receptors, GPER has seven transmembrane domains across the cell surface (Ji et al., 1998). Early estrogen signaling research noted the ability of estrogen to trigger activation of cAMP, calcium, and other second messengers, but years later researchers discovered GPER, not ESR1/2, was responsible for these responses (Filardo, 2002; Revankar, 2005). In mice, GPER is expressed in a variety of tissues, including arteries, throughout the brain, stomach, the pituitary gland, and the adrenal gland (Isensee et al., 2009). In humans, GPER is expressed in the heart, placenta, lung, kidney, pancreas, spleen, stomach, and small intestine, among others (Olde and Leeb-Lundberg, 2009). In the zebrafish, gper1 exhibits a similar expression pattern in the brain, intestine, muscle tissue, and gonads (Liu et al., 2009; Romano et al., 2017; Chaturantabut et al., 2019). Once again, the function of GPER seems to expand beyond that of traditional reproductive roles that have been previously associated with estrogen signaling.
The zebrafish as a model for developmental biology
Studying developmental processes is a complex undertaking, especially in the case of mammalian models. Murine ontogeny, for example, occurs in utero over the course of a few weeks, preventing rapid live imaging experimentation. The zebrafish offer a solution to these barriers, as fertilization occurs ex utero, and animals maintain optical transparency through early developmental stages. These features allow for high-throughput genetic manipulation studies via microinjection coupled with real-time visualization of morphogenesis. Gastrulation begins at just 5 hours post fertilization (hpf) with organ formation initiating by 10 hpf (Lieschke and Currie, 2007; Kimmel et al., 1995). This makes the zebrafish highly amenable for studying the workings of organogenesis. In addition, the genes controlling these processes are highly conserved, with over 80% of human disease-associated genes having a zebrafish orthologue (Howe et al., 2013). Zebrafish also thrive in a laboratory setting and can be well cared for at a reasonable cost, which has made them a popular developmental model over the past several decades (Lieschke and Currie, 2007; Kimmel et al., 1995).
Conservation of organ anatomy: the case of kidney segmentation
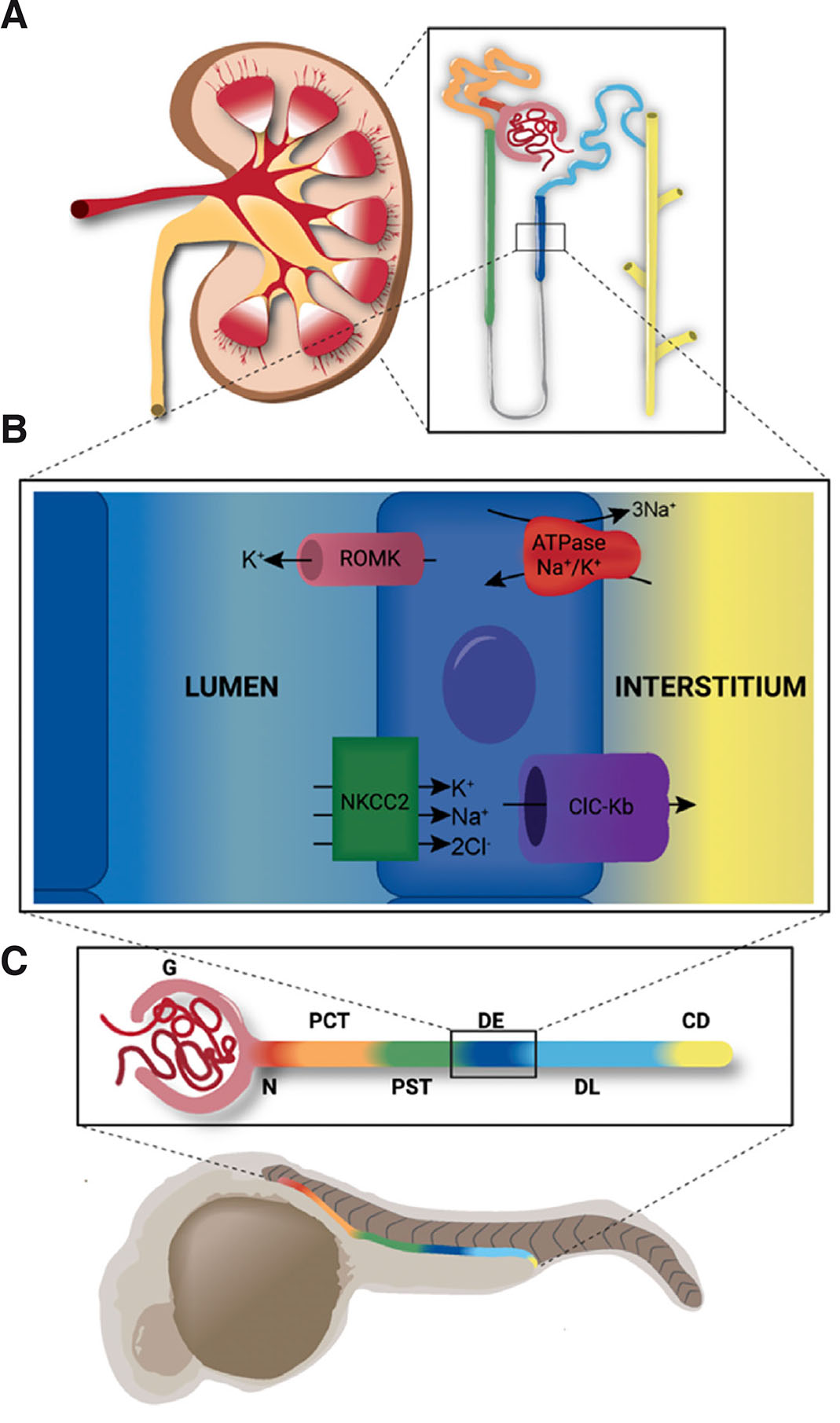
Vertebrate organ anatomy is highly conserved between zebrafish and mammals. For example, both form kidneys that are comprised of structural and functional units known as nephrons (Gerlach and Wingert, 2013). Furthermore, nephrons exhibit similar compositions, with a blood filter followed by a tubule that reabsorbs and secretes solutes. Nephron tubules are similarly subdivided into functional zones of specialized cells known as segments that perform discrete physiological tasks in modifying the filtrate (Fig. 2) (Wingert et al., 2007; Wingert and Davidson, 2011). The proximal segments are responsible for most reabsorption activities, such as uptake of glucose and amino acids, though they also coordinate acid-base homeostasis and drug secretion. The distal segments are responsible for fine tuning salt levels and other electrolytes. Of course, differences do exist in organ structure between species; a notable difference with respect to the kidney is the presence or absence of the loop of Henle, which is responsible for water conservation. This is missing in zebrafish which live in freshwater and do not concentrate their urine.
Fig. 2. Nephron segmentation is conserved across vertebrates.
Zebrafish organs exhibit fundamentally similar structure and function to those of mammals. (A) The fully formed mammalian metanephric kidney is comprised of hundreds of thousands of nephrons (inset). (B) The thick ascending limb of the mammalian kidney is analogous to the distal early segment of the zebrafish. These segments share similar functions and express the same suite of solute transporters, including ROMK (encoded by kcnj1a1.1 in zebrafish), ATPase Na+/K+ (encoded by atp1a1a.1 in zebrafish), NKCC2 (encoded by slc12a1 in zebrafish), and ClC-Kb (encoded by clcnk in zebrafish). (C) The zebrafish pronephros shares similar segmentation with the mammalian nephron (color-coding). The pronephros is comprised of the glomerulus (G), neck (N), proximal convoluted tubule (PCT), proximal straight tubule (PST), distal early (DE), distal late (DL) and collecting duct (CD).
While the nephron is organized into these specialized segments, recent RNA sequencing studies have provided a new appreciation for the complexity of these cell types (Tang et al., 2017; Liao et al., 2020; McEvoy et al., 2022; Ransick et al., 2019). This complexity is further amplified in the context of various disease states and cancers (Hinze et al., 2022; Zhang et al., 2021). The zebrafish, as a high throughput and conserved model, is particularly useful in interrogating genes of interest from these large datasets (Naylor et al., 2017). Through various chemical and genetic screens, researchers have garnered new insights to the regulatory networks controlling nephrogenesis (Chambers et al., 2023; Nguyen et al., 2023).
Roles of estrogen and ERs in organ development revealed by zebrafish studies
In the subsections that follow, we discuss how the zebrafish has been utilized to discover new insights into the roles of estrogen signaling during development of the kidney, gonads, brain, blood and finally, the liver.
Kidney
Using the zebrafish as a model to investigate developmental pathways of kidney formation, chemical screens have identified several chemical regulators of interest, including estrogen signaling (Poureetezadi et al., 2016; Wesselman et al., 2023). Identifying a link between estrogen signaling and nephron development was surprising, as estrogen has traditionally been considered a sex hormone, with most research focusing on gonad development. Specifically, a screen of bioactive chemicals first revealed that E2 resulted in changes in nephron segmentation (Poureetezadi et al., 2016). While expression of specific estrogen receptors has not been detected in the pronephros by whole mount in situ hybridization, a transgenic line showing activation of the ERE indicates that estrogen signaling is active in the distal pronephros (Carroll et al., 2014). This data suggests that ligand activated transcription factors, Esr1, Esr2a, and Esr2b, are of particular interest in the context of nephrogenesis.
In our study, we found that exogenous treatment of E2 resulted in specific changes to the distal pronephros, where the DE was increased at the expense of the neighboring DL segment (Wesselman et al., 2023). A selective estrogen receptor modulators (SERM) screen revealed that this phenotype was largely due to Esr2 activity, as inhibition with PHTPP resulted in the opposite phenotype (Wesselman et al., 2023b). One caveat to the chemical data is that ligand specificity of PHTPP for Esr2 has not been measured in zebrafish cells specifically. However, the PHTPP results were recapitulated with morpholino experiments, as only esr2b knockdown, not esr2a nor esr1, resulted in a decreased DE and increased DL (Wesselman et al., 2023). Further analysis revealed that E2 signaling mediated by Esr2b operates upstream of essential distal transcription factors Irx3b and its target Irx1a (Wesselman et al., 2023). Taken together, Esr2b seems to regulate cell fate choice in multiple zebrafish tissues, as a similar phenotype was observed in liver tissue (Wesselman et al., 2023; Chaturantabut et al., 2020).
Reproductive system
Zebrafish offer a unique perspective to studying estrogen signaling, as adults exhibit sex plasticity in mature gonads, which has been shown using treatment of androgens and aromatase inhibitors (Lee et al., 2017; Takatsu et al., 2013). Exogenous estrogen treatment throughout development results in all offspring becoming female, but some oocytes are unable to reach full maturation (Fenske et al., 2005). Interestingly, removal of exogenous estrogen results in a more balanced population, where some of the animals become male (Fenske et al., 2005). With a combination of xenoestrogens, males exhibit a decreased sperm count, but there is also an increase in the number of proliferating germ cells (Wang et al., 2019). Interestingly, estrogen exposure alone promotes proliferation of undifferentiated spermatogonia but depletes meiotic and post meiotic germ cells (de Castro Asiss et al., 2018). Together, these studies suggest the potency of estrogen in sex determination of zebrafish, as well as its important in gonad function.
In loss of function studies of each of the estrogenic nuclear receptors, single mutants of esr1, esr2a, or esr2b exhibit normal reproductive development (Lu et al., 2017). In females, double knockout of esr2a and esr2b results in dysfunctional follicular formation and a gonad reprogramming to male (Lu et al., 2017). Similarly, knockout of gper1 results in reduced fertility in females (Wu et al., 2021). Together, this suggests that the estrogen receptors have some functional redundancy, especially in reproductive development.
Brain
Zebrafish have two isoforms of aromatase, encoded by cyp19a1a and cyp19a1b, which are known as ovarian aromatase and brain aromatase, respectively. Both forms are present in early development, as they decrease shortly after fertilization, followed by a drastic increase of cyp19a1b at 24 hours (Sawyer et al., 2006). This increase is likely due to the depletion of the maternally deposited E2 in the yolk through development, resulting in activation of the endogenous synthesis program. In the brain specifically, E2 has been shown to decrease cell proliferation and cell migration in the adult zebrafish, which differs from responses observed in mammalian models (Makantasi and Dermon, 2014; Diotel et al., 2013). In the context of development, low dose exposure to E2 increases serotonin transmission by serotonergic neurons while high dose exposure had the opposite effect (Ulhaq and Kishida, 2018). Further, knockdown of brain aromatase specifically decreased serotonin levels, revealing its necessity for serotonergic neuron development in zebrafish, which in turn are required to control heart rate and anxiety behavior in embryos (Ulhaq and Kishida, 2018). Specific inhibition of gper1 resulted in growth retardation of the brain, with increased apoptosis, decreased proliferation of sensory and motor neurons (Shi et al., 2013). GPER mutants also exhibit decreased triiodothyronine (T3) levels, which is an essential thyroid hormone needed to modulate heart rate (Romano et al., 2017). Interestingly, GPER specifically regulates maternal estrogen levels as well as T3 levels, which results in regulation of the embryonic heart rate (Romano et al., 2017). Together, these studies point to the importance of balance of estrogenic hormones, as both exogenous E2 and inhibition of E2 signaling can result in alterations to neurogenesis and subsequent brain function.
Hematopoiesis
Although hematopoietic stem cells (HSC) were not considered sexually dimorphic, recent research has found that mouse HSCs exhibit sex differences in cell cycle regulation (Nakada et al., 2014). Researchers discovered that E2 induces HSC division in both males and females, and that deletion of Esr1 from HSCs reduces divisions in females but not males (Nakada et al., 2014). Similarly, another study found that Esr1 is not required for the production of normal HSC proportions in males, but is involved in B cell regulation (Thurmond et al., 2000). E2 has also been shown to modulate the HSC niche in zebrafish by setting the ventral boundary of the hemogenic vascular niche (Carroll et al., 2014). Interestingly, treatment with exogenous E2 or xenoestrogens (ethinylestradiol and genistein) reduces HSC formation, while inhibition of E2 activity via chemical treatment (pan-inhibitor ZK164015 or Esr2 inhibitor PHTPP) or double knockdown of esr2a and esr2b increases runx1/cmyb expression, which mark HSPCs (Carroll et al., 2014). These alterations in HSC formation are linked to malformation of the hemogenic endothelial niche and modulation of VEGF signaling (Carroll et al., 2014). These data warrant further investigation into the developmental effects of estrogenic exposure, and may offer insight to sex differences observed in HSC regulation.
Liver
All four estrogen receptors of the zebrafish are expressed in the liver at varying time points (Menuet et al., 2002; Bertrand et al., 2007; Romano et al., 2017; Chaturantabut et al., 2019). For example, the adult liver expresses esr1, esr2a and esr2b as assessed by reverse transcription polymerase chain reaction (Menuet et al., 2002). In the developing zebrafish liver, esr2b is detectable by whole mount in situ hybridization (WISH) at 72 hpf, but decreases shortly thereafter (Bertrand et al., 2007; Chaturantabut et al., 2020). By 96 hpf, gper1 is robustly expressed in the liver, as detected by WISH (Chaturantabut et al., 2019). This dynamic expression is in line with the phases of liver formation, as specification occurs from 6 hpf to 30 hpf, followed by budding and differentiation from 32 hpf to 72 hpf, and finally by outgrowth after 72 hpf (Chu and Sadler, 2009).
In the early stages of development, E2 treatment prior to 72 hpf results in decreased hepatocyte number and overall liver size, while blockade of estrogen signaling increases liver size (Chaturantabut et al., 2020). This phenotype was linked to esr2b, as mutants had robustly increased hepatocyte marker expression, at the expense of biliary lineages (Chaturantabut et al., 2020). Decisions between biliary and hepatocyte cell fate were indeed mediated by Esr2b signaling and occurred downstream of BMP signaling (Chaturantabut et al., 2020). Interestingly, the opposite was true in larval and adult animals, as exogenous E2 treatment increased liver size of both larval (over 120 hpf) and adult zebrafish, though this was due to proliferation rather than cell fate (Chaturantabut et al., 2019). An SERM screen revealed that the main driver of this phenotype was Gper1, as inhibition via G-15 treatment or morpholino knockdown resulted in the opposite phenotype (Chaturantabut et al., 2019). Additional cell cycle analysis of liver cells in 120 hpf animals revealed that E2 drives hepatocyte proliferation, cell cycle progression and liver size, and all phenotypes were diminished in animals lacking gper1 expression (Chaturantabut et al., 2019). Additional epistatic experiments revealed that PI3K-Akt pathway operates downstream of Gper1 mediated E2 signaling, and subsequently activates mTOR signaling in the context of regeneration (Chaturantabut et al., 2019). The same pro-proliferative effect of E2 was similarly observed in primary hepatocyte culture and in liver cancer (Chaturantabut et al., 2020). Together, these data point to the importance of E2 modulation in both development and adulthood, and that temporal regulation of estrogen signaling occurs via different receptors.
Conclusions and future prospects
During vertebrate development, estrogen levels are highly regulated. The importance of this tight regulation is highlighted by the potent consequences of estrogen signaling on various developmental tissues during embryogenesis, as discussed here with respect to formation of mesoderm, endoderm and ectoderm derivatives. There continue to be new insights as researchers conduct further studies, as in the case of the brain, where estrogen activity was recently linked to proper development of the olfactory sensory system (Takesono et al., 2022). In that case, inhibition of estrogen activity with the estrogen receptor antagonist ICI182 led to morphological disruptions in synaptic development of olfactory neurons and their interacting glia and led to altered behavior in larval stages (Takesono et al., 2022).
Environmental estrogens may not just impact estrogen signaling during gestation, but also during the early stages of life (Hsu and Tain et al., 2021). For example, work with zebrafish has revealed how bisphenol A exposure can lead to alterations in the juvenile brain including neuron loss, activation of microglia, and depressed neurogenesis, which are correlated with the emergence of social behavior defects (Mu et al., 2023; Yang et al., 2023). Others have reported that bisphenol A exposure also leads to disruptions in the ways that sensory information is processed (Scaramella et al., 2022). Understanding how endogenous and exogenous estrogen exposure impacts development and maturation of various tissues will offer valuable insight into the potential effects of various teratogens. With the help of the zebrafish and other models, our appreciation for the importance of hormone signaling will continue to grow.
Acknowledgements
We thank the members of our research lab, most especially Allison Gatz, Liana Arceri, and Mairead Pfaff, for their collaboration in studying estrogen signaling during kidney development.
Declarations
Author contribution
HMW conceived and wrote the first manuscript. HMW and RAW discussed and revised the manuscript.
References
Amenyogbe E., Chen G., Wang Z., Lu X., Lin M., Lin A. Y. (2020). A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. International Journal of Endocrinology 2020: 1-9.
Baker M. E. (2013). What are the physiological estrogens?. Steroids 78: 337-340.
Bertrand S., Thisse B., Tavares R., Sachs L., Chaumot A., Bardet P.L., Escrivà H., Duffraisse M., Marchand O., Safi R., Thisse C., Laudet V. (2007). Unexpected Novel Relational Links Uncovered by Extensive Developmental Profiling of Nuclear Receptor Expression. PLoS Genetics 3: e188.
Bondesson M., Hao R., Lin C.Y., Williams C., Gustafsson J. (2015). Estrogen receptor signaling during vertebrate development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1849: 142-151.
Brandenberger A. W. (1997). Tissue Distribution of Estrogen Receptors Alpha (ER- ) and Beta (ER- ) mRNA in the Midgestational Human Fetus. Journal of Clinical Endocrinology & Metabolism 82: 3509-3512.
Carroll K. J., Esain V., Garnaas M. K., Cortes M., Dovey M. C., Nissim S., Frechette G. M., Liu S. Y., Kwan W., Cutting C. C., Harris J. M., Gorelick D. A., Halpern M. E., Lawson N. D., Goessling W., North T. E. (2014). Estrogen Defines the Dorsal-Ventral Limit of VEGF Regulation to Specify the Location of the Hemogenic Endothelial Niche. Developmental Cell 29: 437-453.
Chambers B. E., Weaver N. E., Lara C. M., Nguyen T. K., Wingert R. A. (2023). (Zebra)fishing for nephrogenesis genes. Tissue Barriers In Press: .
Chaturantabut S., Shwartz A., Evason K. J., Cox A. G., Labella K., Schepers A. G., Yang S., Acuña M., Houvras Y., Mancio-Silva L., Romano S., Gorelick D. A., Cohen D. E., Zon L. I., Bhatia S. N., North T. E., Goessling W. (2019). Estrogen Activation of G-Protein–Coupled Estrogen Receptor 1 Regulates Phosphoinositide 3-Kinase and mTOR Signaling to Promote Liver Growth in Zebrafish and Proliferation of Human Hepatocytes. Gastroenterology 156: 1788-1804.e13.
Chaturantabut S., Shwartz A., Garnaas M. K., LaBella K., Li C.C., Carroll K. J., Cutting C. C., Budrow N., Palaria A., Gorelick D. A., Tremblay K. D., North T. E., Goessling W. (2020). Estrogen Acts Through Estrogen Receptor 2b to Regulate Hepatobiliary Fate During Vertebrate Development. Hepatology 72: 1786-1799.
Chu J., Sadler K. C. (2009). New school in liver development: Lessons from zebrafish. Hepatology 50: 1656-1663.
de Castro Assis L. H., de Nóbrega R. H., Gómez-González N. E., Bogerd J., Schulz R. W. (2018). Estrogen-induced inhibition of spermatogenesis in zebrafish is largely reversed by androgen. Journal of Molecular Endocrinology 60: 273-284.
Diotel N., Vaillant C., Gabbero C., Mironov S., Fostier A., Gueguen M.M., Anglade I., Kah O., Pellegrini E. (2013). Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Hormones and Behavior 63: 193-207.
Farooq A. (2015). Structural and Functional Diversity of Estrogen Receptor Ligands. Current Topics in Medicinal Chemistry 15: 1372-1384.
Fenske M., Maack G., Schäfers C., Segner H. (2005). An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio . Environmental Toxicology and Chemistry 24: 1088-1098.
Filardo E. J. (2002). Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. The Journal of Steroid Biochemistry and Molecular Biology 80: 231-238.
Froehlicher M., Liedtke A., Groh K., López-Schier H., Neuhauss S. C.F., Segner H., Eggen R. I.L. (2009). Estrogen receptor subtype β2 is involved in neuromast development in zebrafish (Danio rerio) larvae. Developmental Biology 330: 32-43.
Gerlach G. F., Wingert R. A. (2013). Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. WIREs Developmental Biology 2: 559-585.
Gorelick D. A., Halpern M. E. (2011). Visualization of Estrogen Receptor Transcriptional Activation in Zebrafish. Endocrinology 152: 2690-2703.
Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Ström A., Treuter E., Warner M., Gustafsson J. (2007). Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiological Reviews 87: 905-931.
Hinze C., Kocks C., Leiz J., Karaiskos N., Boltengagen A., Cao S., Skopnik C. M., Klocke J., Hardenberg J.H., Stockmann H., Gotthardt I., Obermayer B., Haghverdi L., Wyler E., Landthaler M., Bachmann S., Hocke A. C., Corman V., Busch J., Schneider W., Himmerkus N., Bleich M., Eckardt K.U., Enghard P., Rajewsky N., Schmidt-Ott K. M. (2022). Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury. Genome Medicine 14: 103.
Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L., et al. (2013). Nature 496: 498-503.
Hsu C.N., Tain Y.L. (2021). Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Frontiers in Endocrinology 12: 745716.
Isensee J., Meoli L., Zazzu V., Nabzdyk C., Witt H., Soewarto D., Effertz K., Fuchs H., Gailus-durner V., Busch D., Adler T., de Angelis M. H., Irgang M., Otto C., Noppinger P. R., (2009). Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150: 1722-1730.
Ji T. H., Grossmann M., Ji I. (1998). G Protein-coupled Receptors. Journal of Biological Chemistry 273: 17299-17302.
Kao R. M., Vasilyev A., Miyawaki A., Drummond I. A., McMahon A. P. (2012). Invasion of Distal Nephron Precursors Associates with Tubular Interconnection during Nephrogenesis. Journal of the American Society of Nephrology 23: 1682-1690.
Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics 203: 253-310.
Kuiper G. G. J. M., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J.A. (1997). Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 138: 863-870.
Lee S. L. J., Horsfield J. A., Black M. A., Rutherford K., Fisher A., Gemmell N. J. (2017). Histological and transcriptomic effects of 17α-methyltestosterone on zebrafish gonad development. BMC Genomics 18: 557.
Liao J., Yu Z., Chen Y., Bao M., Zou C., Zhang H., Liu D., Li T., Zhang Q., Li J., Cheng J., Mo Z. (2020). Single-cell RNA sequencing of human kidney. Scientific Data 7: 4.
Lieschke G. J., Currie P. D. (2007). Animal models of human disease: zebrafish swim into view. Nature Reviews Genetics 8: 353-367.
Liu X., Zhu P., Sham K. W.Y., Yuen J. M.L., Xie C., Zhang Y., Liu Y., Li S., Huang X., Cheng C. H.K., Lin H. (2009). Identification of a Membrane Estrogen Receptor in Zebrafish with Homology to Mammalian GPER and Its High Expression in Early Germ Cells of the Testis1. Biology of Reproduction 80: 1253-1261.
Lu H., Cui Y., Jiang L., Ge W. (2017). Functional Analysis of Nuclear Estrogen Receptors in Zebrafish Reproduction by Genome Editing Approach. Endocrinology 158: 2292-2308.
Luu-The V. (2013). Assessment of steroidogenesis and steroidogenic enzyme functions. The Journal of Steroid Biochemistry and Molecular Biology 137: 176-182.
Makantasi P., Dermon C.R. (2014). Estradiol treatment decreases cell proliferation in the neurogenic zones of adult female zebrafish (Danio rerio) brain. Neuroscience 277: 306-320.
McEvoy C. M., Murphy J. M., Zhang L., Clotet-Freixas S., Mathews J. A., An J., Karimzadeh M., Pouyabahar D., Su S., Zaslaver O., Röst H., Arambewela R., Liu L. Y., Zhang S., Lawson K. A., Finelli A., Wang B., MacParland S. A., Bader G. D., Konvalinka A., Crome S. Q. (2022). Single-cell profiling of healthy human kidney reveals features of sex-based transcriptional programs and tissue-specific immunity. Nature Communications 13: 7634.
Mu X., Liu Z., Zhao X., Chen L., Jia Q., Wang C., Li T., Guo Y., Qiu J., Qian Y. (2023). Bisphenol analogues induced social defects and neural impairment in zebrafish. Science of The Total Environment 899: 166307.
Menuet A., Pellegrini E., Anglade I., Blaise O., Laudet V., Kah O., Pakdel F. (2002). Molecular Characterization of Three Estrogen Receptor Forms in Zebrafish: Binding Characteristics, Transactivation Properties, and Tissue Distributions1. Biology of Reproduction 66: 1881-1892.
Nakada D., Oguro H., Levi B. P., Ryan N., Kitano A., Saitoh Y., Takeichi M., Wendt G. R., Morrison S. J. (2014). Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505: 555-558.
Naylor R. W., Qubisi S. S., Davidson A. J. (2017). Zebrafish Pronephros Development. In Kidney Development and Disease. (Ed. Miller Rachel K.) Springer International Publishing, Cham.
Nguyen T. K., Petrikas M., Chambers B. E., Wingert R. A. (2023). Principles of Zebrafish Nephron Segment Development. Journal of Developmental Biology 11: 14.
Olde B., Leeb-Lundberg L.M. F. (2009). GPR30/GPER1: searching for a role in estrogen physiology. Trends in Endocrinology & Metabolism 20: 409-416.
Poureetezadi S. J., Cheng C. N., Chambers J. M., Drummond B. E., Wingert R. A., (2016). Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development. eLife 5: e17551.
Ransick A., Lindström N. O., Liu J., Zhu Q., Guo J.J., Alvarado G. F., Kim A. D., Black H. G., Kim J., McMahon A. P. (2019). Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Developmental Cell 51: 399-413.e7.
Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005). A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 307: 1625-1630.
Romano S. N., Edwards H. E., Souder J. P., Ryan K. J., Cui X., Gorelick D. A. (2017). G protein-coupled estrogen receptor regulates embryonic heart rate in zebrafish. PLOS Genetics 13: e1007069.
Sawyer S. J., Gerstner K. A., Callard G. V. (2006). Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: Gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. General and Comparative Endocrinology 147: 108-117.
Scaramella C., Alzagatiti J. B., Creighton C., Mankatala S., Licea F., Winter G. M., Emtage J., Wisnieski J. R., Salazar L., Hussain A., Lee F. M., Mammootty A., Mammootty N., Aldujaili A., Runnberg K. A., Hernandez D., Zimmerman-Thompson T., Makwana R., Rouvere J., Tahmasebi Z., Zavradyan G., Campbell C. S., Komaranchath M., Carmona J., Trevitt J., Glanzman D., Roberts A. C. (2022). Bisphenol A Exposure Induces Sensory Processing Deficits in Larval Zebrafish during Neurodevelopment. eneuro 9: ENEURO.0020-22.2022.
Shi Y., Liu X., Zhu P., Li J., Sham K. W.Y., Cheng S. H., Li S., Zhang Y., Cheng C. H.K., Lin H. (2013). G-protein-coupled estrogen receptor 1 is involved in brain development during zebrafish (Danio rerio) embryogenesis. Biochemical and Biophysical Research Communications 435: 21-27.
Simpson E.R. (2003). Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology 86: 225-230.
Stanić D., Dubois S., Chua H. K., Tonge B., Rinehart N., Horne M. K., Boon W. C. (2014). Characterization of Aromatase Expression in the Adult Male and Female Mouse Brain. I. Coexistence with Oestrogen Receptors α and β, and Androgen Receptors. PLoS ONE 9: e90451.
Takesono A., Schirrmacher P., Scott A., Green J. M., Lee O., Winter M. J., Kudoh T., Tyler C. R. (2022). Estrogens regulate early embryonic development of the olfactory sensory system via estrogen-responsive glia. Development 149: dev199860.
Takatsu K., Miyaoku K., Roy S. R., Murono Y., Sago T., Itagaki H., Nakamura M., Tokumoto T. (2013). Induction of Female-to-Male Sex Change in Adult Zebrafish by Aromatase Inhibitor Treatment. Scientific Reports 3: 3400.
Tang Q., Iyer S., Lobbardi R., Moore J. C., Chen H., Lareau C., Hebert C., Shaw M.K. L., Neftel C., Suva M. L., Ceol C. J., Bernards A., Aryee M., Pinello L., Drummond I. A., Langenau D. M. (2017). Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. Journal of Experimental Medicine 214: 2875-2887.
Thomas P., Alyea R., Pang Y., Peyton C., Dong J., Berg A.H. (2010). Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids 75: 595-602.
Thurmond T. S., Murante F. G., Staples J. E., Silverstone A. E., Korach K. S., Gasiewicz T. A. (2000). Role of Estrogen Receptor α in Hematopoietic Stem Cell Development and B Lymphocyte Maturation in the Male Mouse 1 . Endocrinology 141: 2309-2318.
Trant J. M., Gavasso S., Ackers J., Chung B.C., Place A. R. (2001). Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry ( Danio rerio ) . Journal of Experimental Zoology 290: 475-483.
Ulhaq Z. S., Kishida M. (2018). Brain Aromatase Modulates Serotonergic Neuron by Regulating Serotonin Levels in Zebrafish Embryos and Larvae. Frontiers in Endocrinology 9: 230.
Wang Y.Q., Li Y.W., Chen Q.L., Liu Z.H. (2019). Long-term exposure of xenoestrogens with environmental relevant concentrations disrupted spermatogenesis of zebrafish through altering sex hormone balance, stimulating germ cell proliferation, meiosis and enhancing apoptosis. Environmental Pollution 244: 486-494.
Wesselman H. M., Gatz A. E., Pfaff M. R., Arceri L., Wingert R. A. (2023). Estrogen Signaling Influences Nephron Segmentation of the Zebrafish Embryonic Kidney. Cells 12: 666.
White R., Lees J. A., Needham M., Ham J., Parker M. (1987). Structural Organization and Expression of the Mouse Estrogen Receptor. Molecular Endocrinology 1: 735-744.
Wingert R. A., Selleck R., Yu J., Song H.D., Chen Z., Song A., Zhou Y., Thisse B., Thisse C., McMahon A. P., Davidson A. J. (2007). The cdx Genes and Retinoic Acid Control the Positioning and Segmentation of the Zebrafish Pronephros. PLoS Genetics 3: e189.
Wingert R. A., Davidson A. J. (2011). Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b . Developmental Dynamics 240: 2011-2027.
Wu X.J., Williams M. J., Kew K. A., Converse A., Thomas P., Zhu Y. (2021). Reduced Vitellogenesis and Female Fertility in Gper Knockout Zebrafish. Frontiers in Endocrinology 12: 637691.
Yang G., Yang L., Liu Q., Zhu Z., Yang Q., Liu J., Beta T. (2023). Protective effects of cyanidin-3-O-glucoside on BPA-induced neurodevelopmental toxicity in zebrafish embryo model. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 264: 109525.
Zhang Y., Narayanan S. P., Mannan R., Raskind G., Wang X., Vats P., Su F., Hosseini N., Cao X., Kumar-Sinha C., Ellison S. J., Giordano T. J., Morgan T. M., Pitchiaya S., Alva A., Mehra R., Cieslik M., Dhanasekaran S. M., Chinnaiyan A. M. (2021). Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proceedings of the National Academy of Sciences 118: e2103240118.